C. elegans SAS-1 ensures centriole integrity and ciliary function, and operates with SSNA-1
C. elegans SAS-1 ensures centriole integrity and ciliary function, and operates with SSNA-1
Gönczy, P.; Jha, K.; Woglar, A.; Busso, C.; Hatzopoulos, G.; Favez, T.
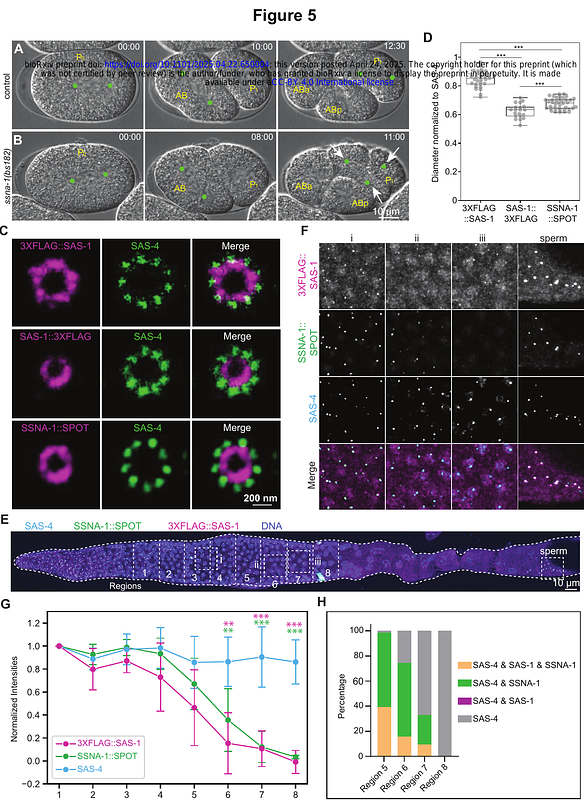
AbstractCentrioles are microtubule-based organelles critical for signaling, motility and division. The microtubule-binding protein SAS-1 is homologous to the human ciliopathy component C2CD3 and contributes to centriole integrity in C. elegans, but how this function is exerted is incompletely understood. Here, through the generation of a null allele and analysis with U-Ex-STED, we establish that SAS-1 is dispensable for the onset of centriole assembly, but essential for organelle integrity during oogenesis, spermatogenesis and in the early embryo. Additionally, we uncover that SAS-1 is present at the transition zone of sensory neurons, where it contributes in a partially redundant manner to ciliary function. Furthermore, we investigate the relationship between SAS-1 and the C. elegans Sjogren Syndrome Nuclear Antigen 1 protein SSNA-1, establishing that SSNA-1 localizes next to the SAS-1 C-terminus in the centriole architecture. Moreover, through molecular epistasis experiments with null alleles of both components, we reveal that SAS-1 is essential for SSNA-1 localization to centrioles during oogenesis and to the transition zone during ciliogenesis. Moreover, using a heterologous human cell assay, we establish that SAS-1 recruits SSNA-1 to microtubules. Overall, our findings help clarify how SAS-1, together with SSNA-1, ensures centriole integrity and reveals that it contributes to cilium function.