Disassembly of the Escherichia coli AcrABZ-TolC efflux pump by ligand-mediated disruption of TolC-AcrA interfacial contacts
Disassembly of the Escherichia coli AcrABZ-TolC efflux pump by ligand-mediated disruption of TolC-AcrA interfacial contacts
Szal, T.; Petsolari, E.; Veliks, J.; Cruz, C. D.; Paunina, L.; Madre, M.; Rachad, F.-Z.; Lewe, P.; Witt, S.; Tammela, P.; Jirgensons, A.; Luisi, B. F.; Windshügel, B.
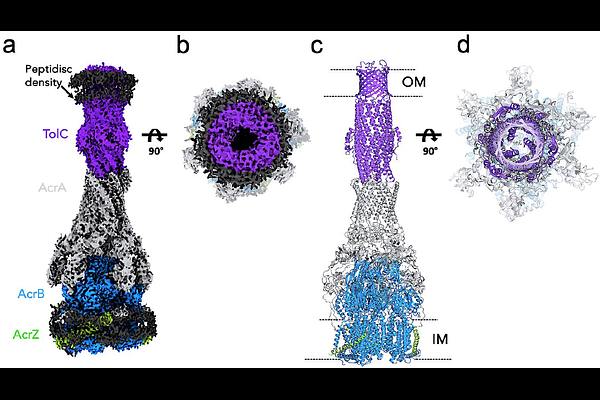
AbstractThe outer membrane factor TolC is an essential component of various efflux pump complexes in E. coli and represents a potential target for antibiotic adjuvants. By means of a virtual screen for TolC-binding compounds, we identified the kinase inhibitor CEP-37440 to shift the minimum inhibitory concentration of antibiotics piperacillin and levofloxacin in E. coli. To determine the substructure relevant for TolC binding, a hit deconstruction approach was applied, resulting in a fragment-like compound with low affinity for TolC and AcrB (LP-115). Dynamic light scattering revealed LP-115 to reduce the hydrodynamic radius of AcrABZ-TolC, indicating a disassembly of the efflux pump complex. A cryo-EM structure demonstrated LP-115 to bind at the TolC-AcrA interface within the AcrABZ-TolC complex, thereby disordering the interface and inducing a closed conformation of TolC. Our results suggest that ligand-mediated TolC-AcrA interface disruption represents a novel mechanism of efflux pump inhibition.