Development and Characterization of a Tunable PDMS Substrate Model for Investigating Elastic Properties and Mechanical Stretching in Intervertebral Disc Mechanobiology
Development and Characterization of a Tunable PDMS Substrate Model for Investigating Elastic Properties and Mechanical Stretching in Intervertebral Disc Mechanobiology
Wuertz-Kozak, K.; Lamoca, M.; Liu, R.; Zhang, S.; Lewis, C. L.; Schimmelpfennig, K.; Hasler, J.
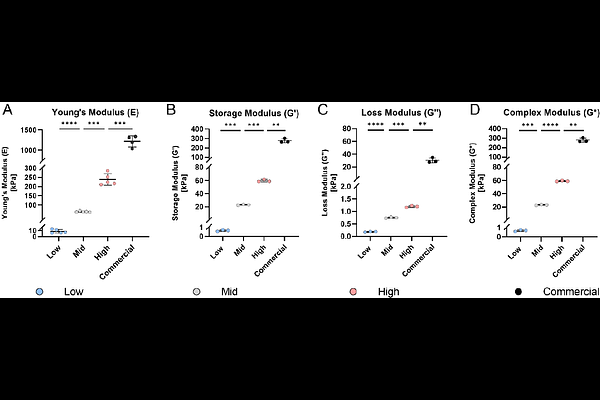
AbstractBackground: Aberrant mechanical loading and altered extracellular matrix (ECM) composition favor catabolic cell responses, contributing to intervertebral disc (IVD) degeneration and ultimately impairing the integrity of the annulus fibrosus (AF). This highlights the need for new in vitro models to investigate the interplay of mechanical loading and cell-substrate interactions. Therefore, this study introduces a tunable stretching chamber platform to simultaneously study both factors in AF degeneration. Methods: Tunable PDMS substrates were fabricated by adjusting ratios of Sylgard 184 and Sylgard 527, enabling molding into stretching chambers or well plates. Substrates underwent mechanical, optical and chemical characterization. Bovine AF cells were seeded onto the substrates and cultured under static conditions or subjected to cyclic strain (8% strain at 1 Hz). Substrate biocompatibility and cell morphology were assessed over 72 h in static cultures. Dynamic responses were assessed by cell viability and alignment. Digital Image Correlation (DIC) was employed to assess surface strain in custom-designed and commercial (STREX) stretching chambers at strains between 8 to 20%. Results: PDMS formulations resulted in a stiffness (E modulus) range of 8.72 to 238.00 kPa, demonstrating distinct viscoelastic profiles. All substrate formulations supported AF cell adhesion and viability. DIC analysis revealed non-uniform axial and transverse strain distributions on membrane surfaces. Cyclic stretching showed that cells maintained viability up to 14 h. Additionally, cells responded to the applied strain by perpendicular alignment to the stretch axis at 6 h. Conclusions: The PDMS based stretching platform offers a biocompatible and tunable mechanical environment that mimics physiological and pathophysiological elastic properties. It enables systematic investigations on how elastic properties and mechanical strain modulate AF cell behavior in IVD disease progression. Finally, this study raises the awareness of non-uniform and transverse strain components within the stretching chambers and highlights the discrepancy of effective strain transfer to the cell interaction surface.