Atomistic Mechanism of Non-Canonical Voltage Gating in TREK K2P Channels
Atomistic Mechanism of Non-Canonical Voltage Gating in TREK K2P Channels
Sun, H.; Aldakul, Y.; Schewe, M.; Diez, C.; Hwang, S.; Baukrowitz, T.
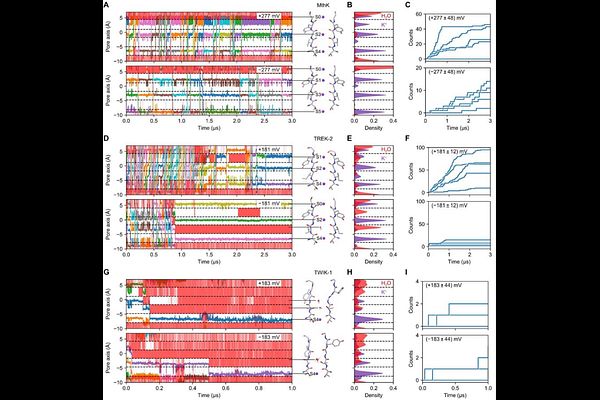
AbstractTwo-pore-domain K+ (K2P) channels are essential regulators of cellular excitability and respond to various external stimuli that also include membrane potential for most K2P channels. Voltage gating is particularly pronounced in members of the TREK/TRAAK subfamily. This voltage sensitivity was surprising, as K2P channels lack a classical voltage sensing domain. Prior studies have attributed this non-canonical voltage sensing mechanism to the unique ion-flux gating properties of the K2P selectivity filter (SF), where inward currents induce fast inactivation, while depolarization activates the SF. Here, we performed large-scale molecular dynamics (MD) simulations across various voltages to gain an atomistic understanding of ion-flux gating in TREK channels. Our analysis revealed an asymmetric stability difference in the SF, enabling water influx into the filter due to conformational flexibility on the extracellular side. Inward flux inactivation occurs when water entry halts ion permeation, followed by the unbinding of three K+ ions, consistent with gating charge analysis. Additionally, MD simulations of TREK-2 mutants and the TWIK-1 channel, which exhibited increased SF flexibility, showed augmented SF water occupancy, aligning with their electrophysiological phenotypes. Key experimental evidence of this mechanism was provided by electrophysiology measurements, which showed that high extracellular sucrose slowed ion-flux inactivation by reducing water entry into the SF. These findings uncover the atomistic mechanism of voltage gating in TREK K2P channels and pave the way for exploring non-canonical voltage gating mechanisms in other ion channels.