Acute temporal, regional, and cell-type specific NKCC1 disruption following severe TBI in the developing gyrencephalic brain.
Acute temporal, regional, and cell-type specific NKCC1 disruption following severe TBI in the developing gyrencephalic brain.
Hochstetler, A. E.; Courtney, Y.; Oloko, P.; Baskin, B.; Ding-Su, A.; Stinson, T.; McGuone, D.; Haynes, R.; Lehtinen, M. K.; Costine-Bartell, B. A.
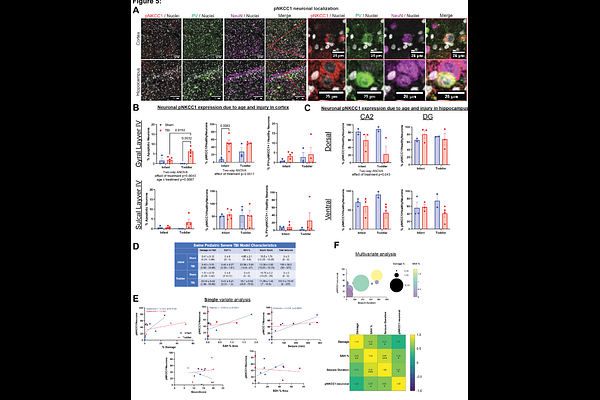
AbstractTraumatic brain injury (TBI) in children is a leading cause of morbidity and mortality, with no effective treatment and limited clinical management. We developed a multifactorial traumatic brain injury model in piglets which mirrors the evolving pathophysiology of severe pediatric TBI, showing age-dependent hypoxic-ischemic cerebral cortical injury and matrix metalloproteinase-driven vasogenic edema, with infant piglets experiencing less tissue damage than toddler piglets. Extracellular matrix breakdown can precipitate neuronal dysfunction, disrupting chloride homeostasis and the reversal potential for GABA. We hypothesized that ongoing tissue damage might be related to markers of immature GABA, evaluated by changes to the expression and phosphorylation of sodium-potassium-2-chloride cotransporter 1 (NKCC1), potassium-chloride cotransporter 2 (KCC2), and a regulatory kinase, (STE20/SPS1-related proline-alanine-rich protein kinase) SPAK. We mapped these markers in developing swine and infant human brain, identifying a postnatal pNKCC1 decrease in human infant hippocampus, and a perinatal cortical and hippocampal GABA switch in pigs, with no change in the thalamus. In infant piglets with severe TBI, upregulation of neuronal pNKCC1 correlated with hypoxic-ischemic injury and seizure duration. We also observed dysregulation of NKCC1, KCC2, and SPAK in cortex and hippocampus in infant and toddler piglets with severe TBI, with thalamus unchanged. We noted ectopic, non-apical localization of pNKCC1 signal in choroid plexus epithelium across ages in piglets and humans with severe TBI, indicating acute dysregulation of the CSF chloride milieu. These findings position swine as a useful model for pediatric TBI research and suggest that SPAK or NKCC1 inhibition in infants may be therapeutic.