Mechanical forces drive mitochondrial matrix extrusion and apoptotic pore growth
Mechanical forces drive mitochondrial matrix extrusion and apoptotic pore growth
Jenner, A.; Dellmann, T.; Kim, H.; Gomez, D.; Zollo, C.; Koefinger, J.; Seidel, K.; Gaedke, F.; Schauss, A.; Hummer, G.; Garcia-Saez, A. J.
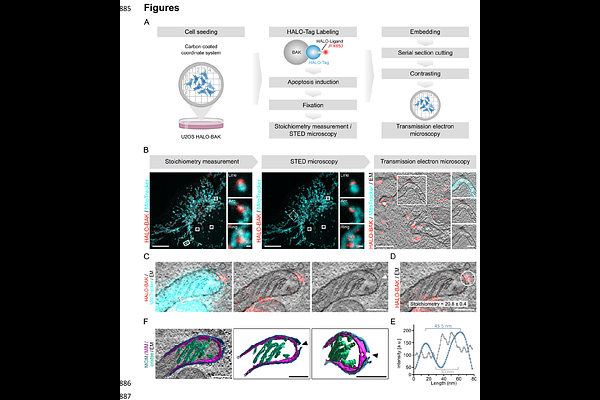
AbstractApoptotic pore opening by BAX and BAK at the mitochondrial outer membrane is a key step in the cell commitment to death. The subsequent inner membrane extrusion and permeabilization releases mitochondrial DNA into the cytosol, which can trigger inflammatory signaling. However, the underlying mechanisms have not been elucidated. Here we developed CLOSE microscopy, a multi-correlative approach that enabled the simultaneous analysis of BAK stoichiometry and nanoscale organization in individual apoptotic pore complexes in relation to mitochondrial ultrastructure. We find that the outer membrane opening at the apoptotic pore defines the spatial arrangement of BAK nanoassemblies. We identify mechanical stress as a driver of inner membrane extrusion, which can be perturbed by osmoregulation. The extruded inner membrane in turn exerts forces on the outer membrane that promote apoptotic pore growth, in line with membrane dynamics simulations. Our study reveals a tight interplay between the inner and outer membranes during mitochondrial permeabilization in apoptosis and establishes a biophysical mechanism for inner membrane extrusion that defines the structural organization of the apoptotic pore.