Ontogeny-independent expression of LPCAT2 in granuloma macrophages during experimental visceral leishmaniasis
Ontogeny-independent expression of LPCAT2 in granuloma macrophages during experimental visceral leishmaniasis
Dey, S.; Cao, J.-H.; Balluff, B.; Dey, N. S.; Gilbert, L.; James, S. R.; Dowle, A.; Calder, G.; O'Toole, P.; Heeren, R. M. A.; Kaye, P. M.
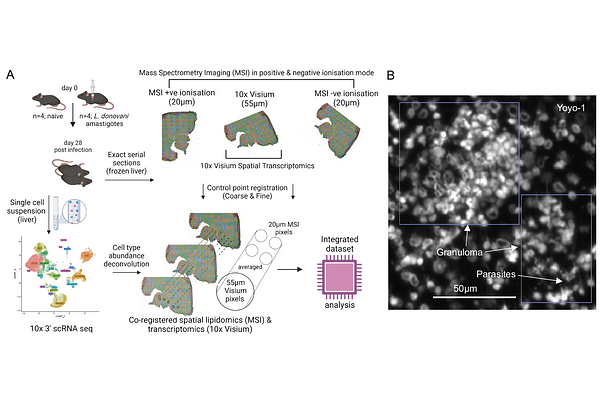
AbstractGranulomas are organized inflammatory lesions that form in response to persistent stimuli such as infections. Murine infection with Leishmania donovani results in the formation of granulomas around infected Kupffer cells in the liver and serves as a well-defined model of immune granuloma formation. The formation and resolution of granulomatous inflammation requires dynamic shifts in immune cell activation states, imposing significant metabolic demands. As mediators of energy homeostasis and cell signaling, lipids and lipid metabolism play a key role in regulating immune cell function during inflammation and the response to infection. However, the extent to which alterations in lipids are spatially linked to altered immune cell transcription has yet to be resolved. In this study, we performed a multimodal imaging analysis combining MALDI mass spectrometry, spatial and single cell transcriptomics, proteomics of flow-sorted macrophages and histopathology of L. donovani induced hepatic granulomas. Using this spatially-integrated approach, we identified LPCAT2-mediated membrane re-modelling of myeloid cells as a novel feature of these granulomas. Our study provides new insights into local immunometabolic changes associated with granuloma formation and macrophage activation.