CCDC32 stabilizes clathrin-coated pits and drives their invagination
CCDC32 stabilizes clathrin-coated pits and drives their invagination
Yang, Z.; Yang, C.; Xu, P.; Han, L.; Li, Y.; Peng, L.; Wei, X.; Schmid, S. L.; Svitkina, T.; Chen, Z.
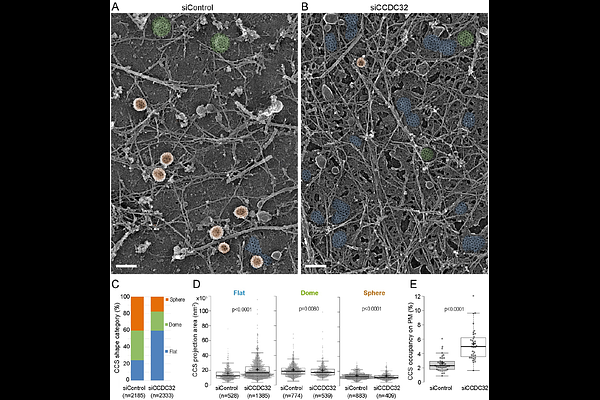
AbstractClathrin-mediated endocytosis (CME) is essential for maintaining cellular homeostasis. Previous studies have reported more than 50 CME accessory proteins; however, the mechanism driving the invagination of clathrin-coated pits (CCPs) remains elusive. Quantitative live cell imaging reveals that CCDC32, a poorly characterized endocytic accessory protein, regulates CCP stabilization and is required for efficient CCP invagination. CCDC32 interacts with the -appendage domain (AD) of AP2 via its coiled-coil domain to exert this function. Furthermore, we showed that the clinically observed nonsense mutations in CCDC32, which result in the development of cardio-facio-neuro-developmental syndrome (CFNDS), inhibit CME by abolishing CCDC32-AP2 interactions. Overall, our data demonstrates the function and molecular mechanism of a novel endocytic accessory protein, CCDC32, in CME regulation.