The genetic architecture of quantitative variation in the self-incompatibility response within Phlox drummondii (Polemoniaceae)
The genetic architecture of quantitative variation in the self-incompatibility response within Phlox drummondii (Polemoniaceae)
Burgin, G. A.; Roda, F.; Farnitano, M.; Hale, C.; Serrato-Capuchina, A.; Hopkins, R.
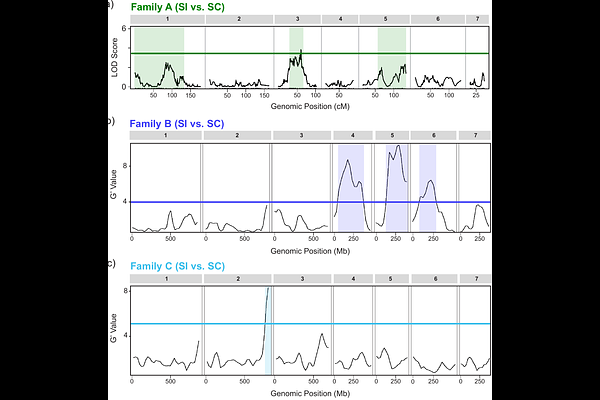
AbstractFlowering plants display extensive variation in selfing rate, a trait with significant ecological and evolutionary consequences. Many species use genetic mechanisms to recognize and reject self-pollen (termed self-incompatibility or SI), and the loss of SI is one of the most common evolutionary transitions among flowering plants. Despite the ubiquity of transitions to self-compatibility (SC), little is known about the genetic architecture through which SC evolves. Specifically, it is important to determine if SC has a polygenic or simple genetic basis and if variation in compatibility localizes to the genomic locus causing self-pollen recognition (the S-locus). Phlox drummondii (Polemoniaceae) has been a model system for exploring mating system evolution and expresses extensive range-wide variation in the SI response. Here we investigate the genetic architecture of SC variants segregating within this otherwise SI species. Using multiple independent crosses, we uncover numerous QTLs associated with intraspecific variation in SI, consistent with a polygenic genetic architecture. While some QTLs overlap across mapping experiments, other QTLs are unique, suggesting that multiple genetic routes to SC exist. Through these crossing experiments, we demonstrate that P. drummondii has a sporophytic SI system, suggesting that an independent evolution of SI occurred in the lineage containing Phlox. We map this novel S-locus and find that the genomic region containing the S-locus is associated with intraspecific variation in SI in one of the three mapping populations. Although further work is necessary to clarify the conditions under which quantitative variation in SI represents a transitional pathway to complete SC, our study reveals the underlying genetic architecture upon which selection could act to drive this frequent and evolutionarily significant transition.