Functional characterization of two glycosyltransferases from Withania somnifera illuminates their role in withanosides biosynthesis and defence against bacteria
Functional characterization of two glycosyltransferases from Withania somnifera illuminates their role in withanosides biosynthesis and defence against bacteria
Anjali, P.; Narayanan, A. K.; Parihar, D.; Patil, A.; Nagegowda, D. A.
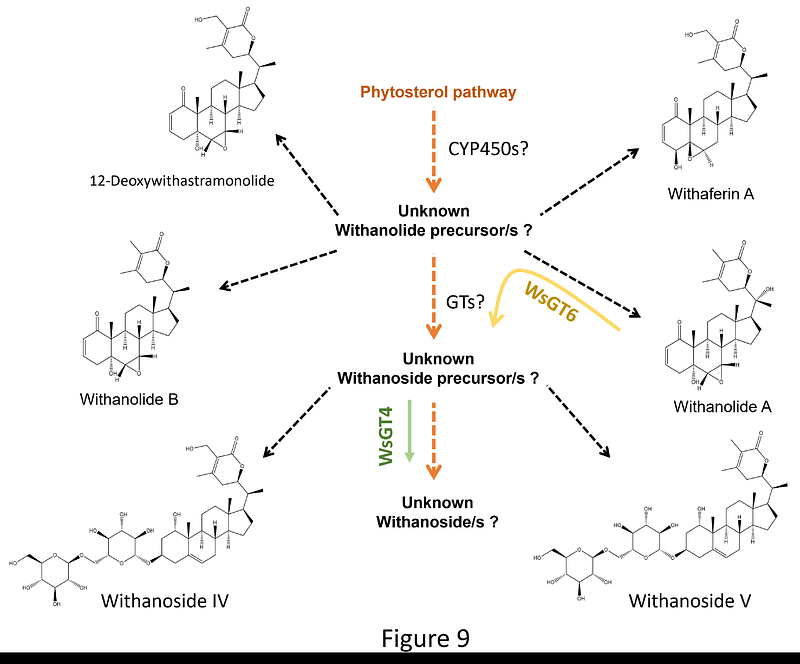
AbstractThe medicinal properties of Ashwagandha (Withania somnifera L. Dunal) are attributed to the presence of unique class of natural products called as withanolides and their glycosylated forms, withanosides. Withanosides are proposed to be formed from withanolides by the action of glycosyltransferases (GTs). This study reports the functional characterization of two GTs (WsGT4 and WsGT6) from W. somnifera that exhibited induced expression in response to methyl jasmonate treatment and showed highest expression in leaves compared to other tissues. Biochemical assays with recombinant WsGT proteins showed that WsGT4 and WsGT6 formed glycosylated products with four and one of the seven tested withanolides substrates, respectively. WsGT4 catalyzed product formation using withanolide A, withanolide B, withanone, and 12-deoxywithastramonolide as substrates, with UDP-glucose serving as the glucose donor, while WsGT6 catalyzed the product formation only with withaferin A as substrate employing either UDP-glucose or UDP-galactose as sugar donors. Moreover, in planta studies through virus-induced gene silencing and transient overexpression of WsGT4 and WsGT6 in W. somnifera leaves modulated the levels of withanolides and withanosides, indicating their role in withanosides biosynthesis. Furthermore, while individual silencing of both WsGT4 and WsGT6 in W. somnifera reduced the tolerance to Pseudomonas syringae DC3000 growth, their overexpression enhanced the tolerance to the bacterium in W. somnifera. Taken together, these results shed light on the roles of WsGT4 and WsGT6 in withanoside biosynthesis and defence against model bacterial pathogen in W. somnifera.