When a chaotropic agent turns into a nutrient: Deciphering the assimilation of guanidine and its utilization to drive synthetic processes in cyanobacteria
When a chaotropic agent turns into a nutrient: Deciphering the assimilation of guanidine and its utilization to drive synthetic processes in cyanobacteria
Itzenhaeuser, M. A.; Enkerlin, A. M.; Dewald, J. A.; Stauder, R.; Halpick, H.; Schaale, R.; Baumann, L. M.; Selim, K. A.; Weinberg, C. E.; Klaehn, S.
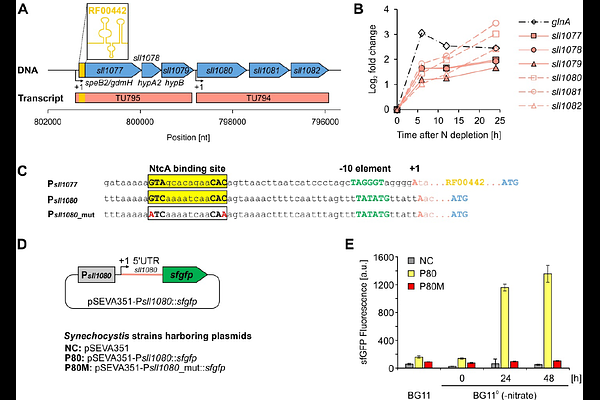
AbstractGuanidine is a well-known chaotropic agent used to denature proteins and nucleic acids. However, recent studies have demonstrated both the widespread synthesis of guanidine, e.g. in plants and mammals, as well as the widespread occurrence of guanidine metabolism in bacteria, suggesting a broader biological role. Here, we provide insights into guanidine assimilation via guanidine hydrolases (GdmH) in cyanobacteria. The gdmH gene is widespread among cyanobacteria and enables growth on guanidine as sole nitrogen source. Strains lacking gdmH, naturally or by deletion, failed to grow on guanidine. Expression of gdmH increased under nitrogen limitation, regulated by the transcription factor NtcA. However, guanidine is toxic above 5 mM, necessitating GdmH activity and adaptive mutations activating the multidrug efflux system PrqA. The gdmH gene is frequently co-localized with ABC transporter genes, which are driven by an additional NtcA-regulated promoter. At low guanidine concentrations, their mutation disrupted guanidine-dependent growth of Synechocystis sp. PCC 6803, supporting that they encode a high affinity transport system. In presence of >1 mM guanidine, these mutants grew like wildtype, suggesting the existence of additional uptake mechanisms for guanidine. We next demonstrate the high-affinity binding of guanidine to a previously described, conserved RNA motif located within the gdmH 5\' UTR, validating it as a guanidine I riboswitch. By combining it with various promoters, we achieved precise, titratable control of heterologous gene expression in cyanobacteria in vivo. Our findings establish guanidine assimilation as an integral element of cyanobacterial nitrogen metabolism and highlight guanidine riboswitches as valuable tools for synthetic biology.