Acyl-coA binding protein AcbdA regulates peroxisome hitchhiking on early endosomes
Acyl-coA binding protein AcbdA regulates peroxisome hitchhiking on early endosomes
Driscoll, B.; Fountain, M. B.; Gates, I. N.; Abdollahi, R.; Langley, A. M.; Owens, M. B.; Christensen, J. R.; Salogiannis, J.
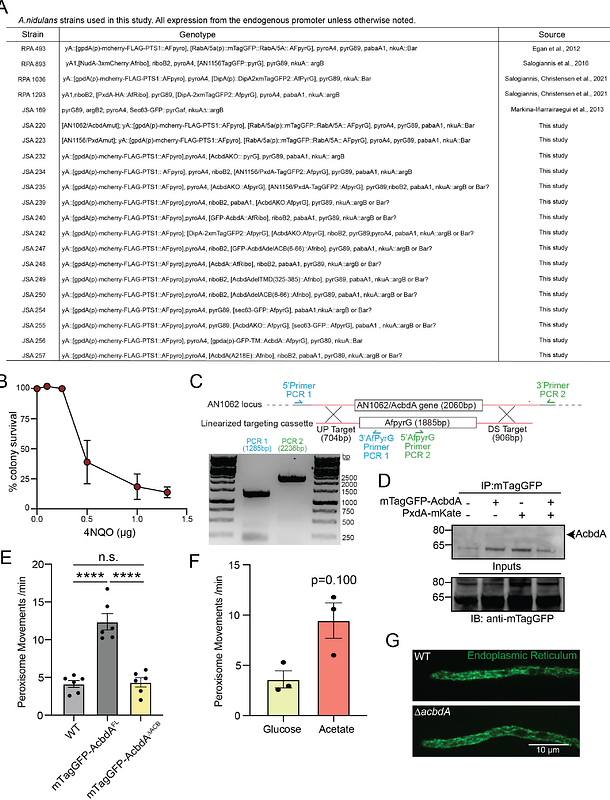
AbstractMotor-driven transport on microtubules is critical for distributing organelles throughout the cell. Most commonly, organelle movement is mediated by cargo adaptors, proteins on the surface of an organelle that directly recruit microtubule-based motors. An alternative mechanism called hitchhiking was recently discovered: some organelles move, not by recruiting the motors directly, but instead by using membrane contact sites to attach to motor-driven vesicles and hitchhike along microtubules. Organelle hitchhiking is observed across fungi and animals. In filamentous fungi, nearly all peroxisomes move by hitchhiking on early endosomes (EEs). In the fungus Aspergillus nidulans, EE-associated linker proteins PxdA and DipA are critical for establishing EE-peroxisome membrane contact sites required for peroxisome movement. How peroxisomes recognize this subset of EEs and what peroxisome-membrane proteins exist that can interact with EEs is not known. Here, we undertook a forward mutagenesis screen to identify such proteins. We discovered an acyl-coA binding (ACB) domain-containing protein AcbdA/AN1062 that localizes to peroxisomes via its tail-anchored transmembrane domain (TMD). Deleting the AcbdA gene or only its N-terminal ACB domain perturbs the movement and distribution of peroxisomes. Importantly, AcbdA is not required for the movement of EEs or for the recruitment of PxdA and DipA on EEs. Fatty acid (FA)-induced increases in peroxisome movement require AcbdA, suggesting that peroxisome hitchhiking on EEs is coupled to FA metabolism. Mutating a conserved FFAT motif, predicted to interact with the endoplasmic reticulum (ER), has no effect on peroxisome movement. Taken together, our data indicate that AcbdA is a peroxisome-membrane protein required to tether peroxisomes to EEs during hitchhiking. AcbdA involvement in peroxisome-EE contact site formation represents a divergence from known functions of Acbd4/5 proteins and adds layers to our understanding of the functionality of the Acbd4/5 family of proteins.