Client-scaffold interactions suppress aggregation of a client protein in model condensates
Client-scaffold interactions suppress aggregation of a client protein in model condensates
Ahmed, R.; Hudson, R. P.; Forman-Kay, J. D.; Kay, L. E.
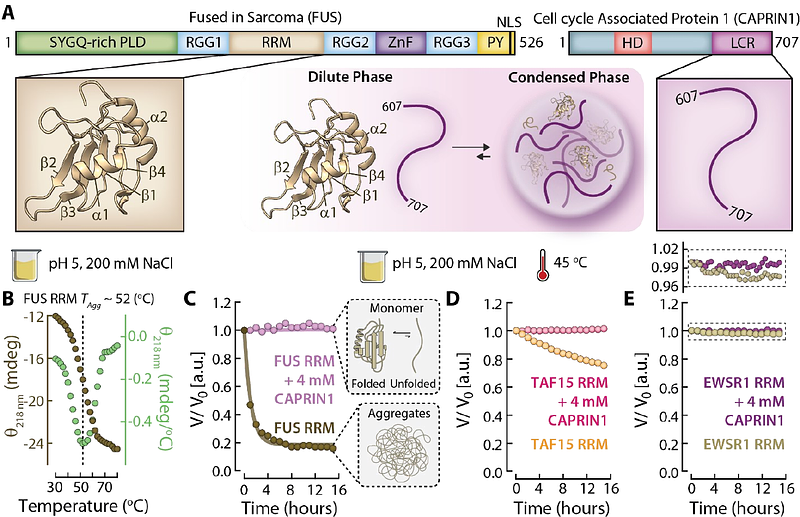
AbstractMany studies have shown that sequestration of client proteins into condensates locally increases their concentrations and/or modulates their conformational landscapes to promote aberrant aggregation. Far fewer examples have emerged where the proteinaceous condensed phase environment protects clients from aggregation. Here, we show that a condensate scaffolded by the C-terminal disordered region of Cell Cycle Associated Protein 1 (CAPRIN1) suppresses aggregation of the Fused in Sarcoma (FUS) RNA Recognition Motif (RRM) client, both components of stress granules. Although FUS RRM aggregation is mediated through the unfolded ensemble, comparative NMR studies of the FUS RRM outside and within the condensate establish that CAPRIN1 condensates attenuate FUS RRM aggregation despite locally increasing its concentration by 2-fold and significantly unfolding the domain. Regions of transient intermolecular contacts between unfolded FUS RRM protomers driving aggregation have been identified, including the hydrophobic segments spanning I287-I308 and G335-A369. Intermolecular NOE experiments recorded on the FUS RRM:CAPRIN1 condensate indicate that CAPRIN1 interacts with much of the unfolded FUS RRM, with regions of stronger contacts including the RRM sequences 287IFVQ290, 296VTIES300, 322INLY325, and 351IDWFDG356. These interactions collectively outcompete the homotypic contacts between unfolded FUS RRM clients driving aggregation. Our results demonstrate that condensate scaffold molecules can, in some cases, shield client interprotomer interactions, delaying or completely suppressing their aggregation.