A Plasma Membrane Vesicle Imaging-Based Platform for Studying Membrane Fusion
A Plasma Membrane Vesicle Imaging-Based Platform for Studying Membrane Fusion
Yosibash, I.; Khan, S.; Lichtenstein, A.; Dharan, R.; Vaknin, A.; Daniel, S.; Avinoam, O.; Sorkin, R.
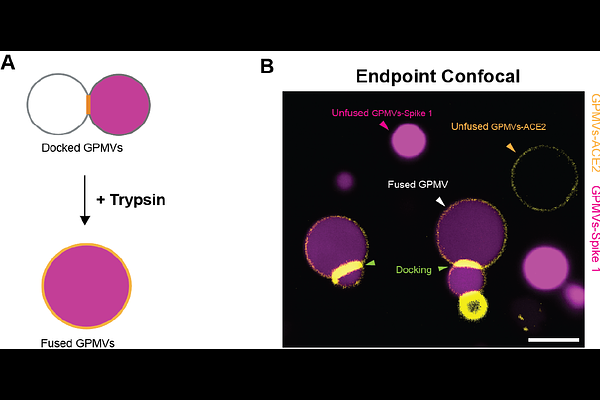
AbstractMembrane fusion is fundamental to biological processes such as fertilization and viral infection. However, studying fusion mechanisms in live cells is challenging due to their complex composition and topography. To address this, we developed a cell-free system based on giant plasma membrane vesicles (GPMVs), enabling us to study the binding and fusion of the SARS-CoV-1 Spike protein with the human ACE2 receptor in a simplified yet physiologically relevant membrane environment. We generated two distinct GPMV populations- one mimicking the viral membrane and the other mimicking the host cell membrane, with the former expressing Spike and cytoplasmic mCherry, and the latter expressing ACE2-GFP. Mixing of these two GPMV populations led to docking of Spike GPMVs onto ACE2 GPMVs. Using live confocal microscopy, we followed and quantified the docking kinetics, confirming receptor accumulation at the docking interface prior to fusion. Fusion was triggered using trypsin, a protease known to activate Spike, and was monitored and quantified using confocal fluorescence microscopy. To quantify fusion efficiency at high throughput, we used Imaging Flow Cytometry. We conclude that the GPMV-based platform offers a versatile tool for dissecting fusion mechanisms beyond viral interactions. By enabling control over experimental conditions, this system provides new opportunities for studying fusion processes with applications in drug delivery, therapeutic design, and fundamental membrane biology research.