Redistribution of fragmented mitochondria ensure symmetric organelle partitioning and faithful chromosome segregation in mitotic mouse zygotes
Redistribution of fragmented mitochondria ensure symmetric organelle partitioning and faithful chromosome segregation in mitotic mouse zygotes
Gekko, H.; Nomura, R.; Kuzuhara, D.; Kaneyasu, M.; Koseki, G.; Adhikari, D.; Mio, Y.; Carroll, J.; Kono, T.; Funahashi, H.; Wakai, T.
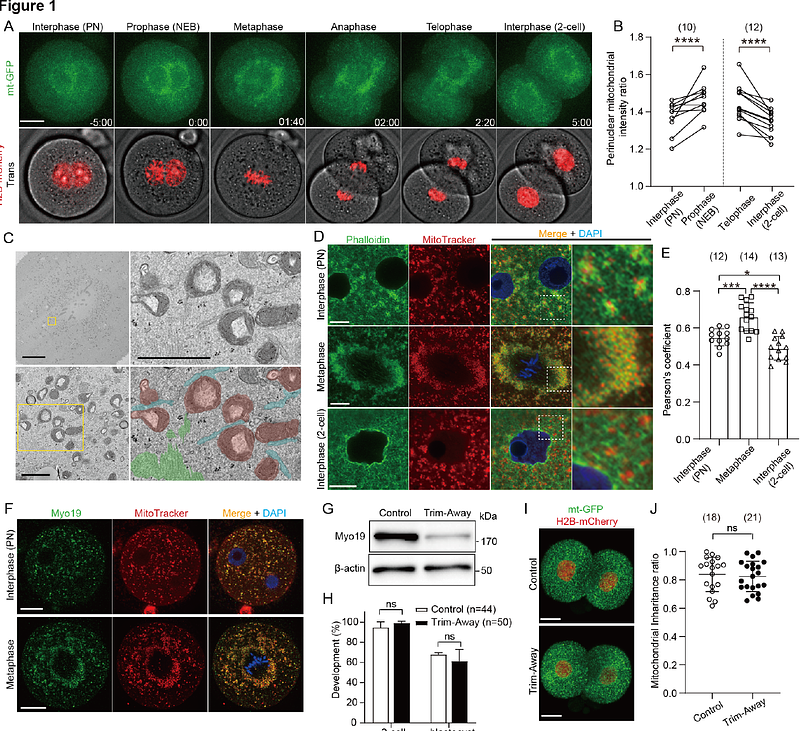
AbstractIn cleavage-stage embryos, pre-existing organelles partition evenly into daughter blastomeres without signi?cant cell growth after symmetric cell division. The presence of mitochondrial DNA within mitochondria and its restricted replication during preimplantation development makes their inheritance particularly important. While chromosomes are precisely segregated by the mitotic spindle, the mechanisms controlling mitochondrial partitioning remain poorly understood. In this study, we investigate the mechanism by which Dynamin-related protein 1 (Drp1) controls the mitochondrial redistribution and partitioning during embryonic cleavage. Deletion of Drp1 in mouse embryos causes marked mitochondrial aggregation, and the majority of embryos arrest at the 2-cell stage. Clumped mitochondria are located in the center of mitotic Drp1-depleted zygotes with less uniform distribution, thereby preventing their symmetric partitioning. Asymmetric mitochondrial inheritance is accompanied by functionally inequivalent blastomeres with biased ATP and endoplasmic reticulum Ca2+ levels. We also find that marked mitochondrial centration in Drp1-depleted zygotes prevents the assembly of parental chromosomes, resulting in chromosome segregation defects and binucleation. Thus, mitochondrial fragmentation mediated by Drp1 ensure proper organelle positioning and partitioning into functional daughters during the first embryonic cleavage.


