Listeria adhesion protein orchestrates caveolae-mediated apical junctional remodeling of epithelial barrier for L. monocytogenes translocation
Listeria adhesion protein orchestrates caveolae-mediated apical junctional remodeling of epithelial barrier for L. monocytogenes translocation
Drolia, R.; Tenguria, S.; Bryant, D.; Thind, J.; Amelunke, B.; Liu, D.; Gallina, N.; Mishra, K.; Samaddar, M.; Sawale, M.; Mishra, D.; Cox, A. D.; Bhunia, A. K.
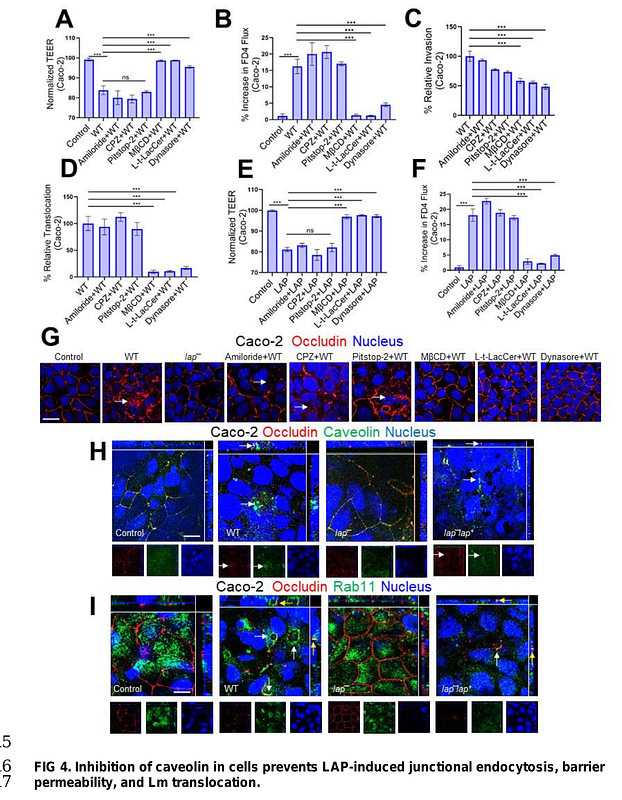
AbstractThe cellular junctional architecture remodeling by LAP-Hsp60 interaction for L. monocytogenes (Lm) passage through the epithelial barrier is incompletely understood. Here, using the gerbil model, permissive to internalin (Inl) A/B-mediated pathways like in humans, we demonstrate that Lm crosses the intestinal villi at 48 h post-infection. In contrast, the single isogenic (lap- or {Delta}inlA) or double (lap-{Delta}inlA) mutant strains show significant defects. LAP promotes Lm translocation via endocytosis of cell-cell junctional complex in enterocytes that do not display luminal E-cadherin. In comparison, InlA-mediated transcytosis occurs in enterocytes displaying apical E-cadherin during cell extrusion and mucus expulsion from goblet cells. LAP hijacks caveolar endocytosis to traffic integral junctional proteins to the early and recycling endosomes. Pharmacological inhibition in a cell line and genetic knock-out of caveolin-1 in mice prevents LAP-induced intestinal permeability, junctional endocytosis, and Lm translocation. Furthermore, LAP-Hsp60-dependent tight junction remodeling is also necessary for InlA access to E-cadherin for Lm intestinal barrier crossing in InlA-permissive hosts.