Chemoenzymatic synthesis of sialylated and fucosylated mucin analogs reveals glycan-dependent effects on protein conformation and degradation.
Chemoenzymatic synthesis of sialylated and fucosylated mucin analogs reveals glycan-dependent effects on protein conformation and degradation.
Kramer, J. R.; Wood, A. M.; Wardzala, C. L.
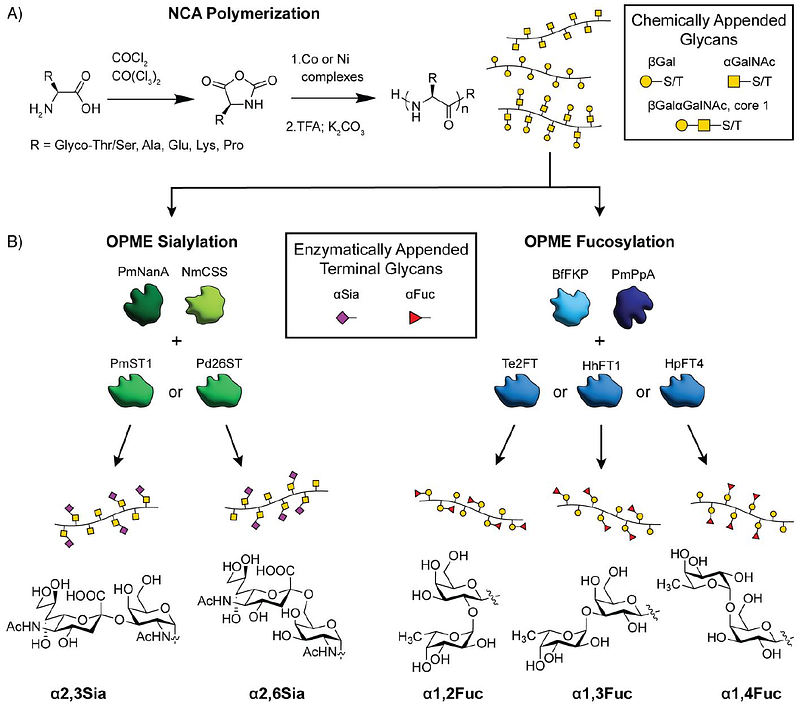
AbstractMucin proteins are essential for life but are challenging to study due to their complex glycosylation patterns. Synthetic mimics have become vital tools for understanding and modulating the roles of mucins in human health and disease. We developed a chemoenzymatic approach to prepare polypeptide-based synthetic mucins displaying a variety of glycans with native linkages and orientations. By combining the polymerization of glycosylated amino acid N-carboxyanhydrides with enzymatic sialylation and fucosylation, we produced a tunable panel of synthetic mucins. These polymers were recognized by natural glycan-binding and glycan-degrading enzymes, providing insights into the structural preferences of these proteins. Glycan- and linkage-dependent effects on proteolysis were observed. Further, investigation of the influence of glycans on peptide backbone secondary structure revealed that both sialylation and linkage at Ser vs. Thr have profound effects on hierarchical conformation. Overall, our methodology offers versatile tools for exploring the diverse glycobiology of mucins.