Injectable borax-loaded alginate hydrogels reduce muscle atrophy, inflammation and generate neuroprotection in the SOD1G93A mouse model of ALS
Injectable borax-loaded alginate hydrogels reduce muscle atrophy, inflammation and generate neuroprotection in the SOD1G93A mouse model of ALS
Rodriguez-Romano, A.; Gonzalez-Valdivieso, J.; Moreno-Martinez, L.; Costa, J. F. V.; Osta, R.; Rico, P.
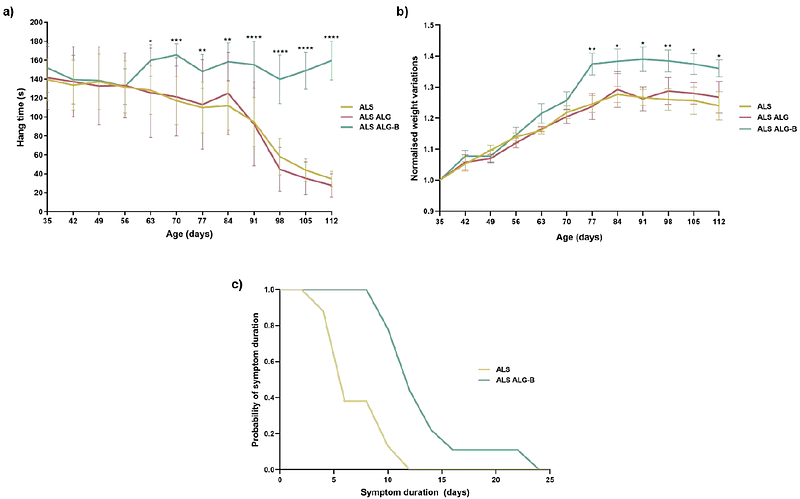
AbstractAmyotrophic Lateral Sclerosis (ALS) is the most frequent and fatal condition that causes motor neurons loss and skeletal muscle paralysis. Although ALS is associated with mutations in over 40 genes, its etiology remains elusive without a cure or effective treatment. Historically considered the prototype of motor neuron diseases, ALS is defined today as a multisystem disorder that presents several changes in non-neuronal cell types, such as pathological changes in muscle occurring before disease onset and independent from motor neuron degeneration (dying back hypothesis). We based in the hypothesis that skeletal muscle may have an active contribution to disease pathology and thus we consider skeletal muscle tissue as a therapeutic target for ALS. In previous works we have demonstrated that borate (B) transporter NaBC1 (encoded by SLC4A11 gene), after activation co-localize with integrins and growth factor receptors producing a functional cluster that synergistically enhances crosstalk mechanisms accelerating muscle repair. In this work we aimed to study the effects of B in a SOD1 mouse model of ALS targeting muscle. We have engineered and characterized injectable alginate-based hydrogels with controlled local borax-release for an effective activation of NaBC1 in vivo. Treated mice presented improved motor function and extended survival that was correlated with an enhanced muscle repair response inducing a reduction in muscle atrophy and inflammation. Interestingly, the activation of muscle repair mechanisms at local level, produced a retrograde neuroprotection by motor neuron preservation and reduction in neuro-inflammation. Altogether, this work presents evidence supporting the involvement of muscle tissue in ALS pathology, reinforcing skeletal muscle as a primary target to develop new therapies for ALS. We propose a novel strategy based on NaBC1 activation for ALS muscle regeneration.