Individualized AI-driven neuromodulation enhances tongue motor and sensory control: preliminary efficacy targeted towards the alleviation of chronic cranial neuropathies
Individualized AI-driven neuromodulation enhances tongue motor and sensory control: preliminary efficacy targeted towards the alleviation of chronic cranial neuropathies
Papageorgiou, T. D.; Webb, J.; Allam, A.; Froudarakis, E.; Rohren, E. M.; Sturgis, E. M.; Hutcheson, K. A.; Heilbronner, S. R.
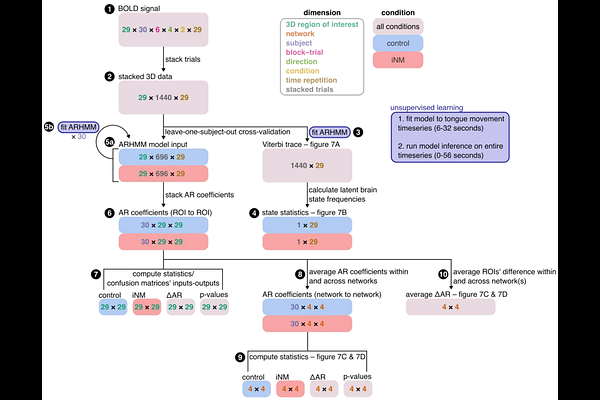
AbstractIntroduction: Adequate sensory inputs and motor control are essential components of safe swallowing, with significant impact on both quality of life and patient survival. Conversely, both of these neuronal activities are negatively impacted during the early phases of ALS development, following instrumentation of the cervical spine through an anterior approach and broadly by delivery of surgical and radiation-based treatments for head and neck cancer. Our goal is to demonstrate the feasibility, and preliminary efficacy of the individualized fMRI neuromodulation (iNM) intervention pioneered by the Papageorgiou Lab (U.S. Patent No.16/954,256; European Patent No. 19 753 851.5) in enhancing tongue motor and sensory control (TMSC) in these variable patient populations. Although clinical trials show that steroids (NCT04151082) and gabapentin (NCT03747562) temporarily reduce pain, their adverse effects compromise tolerance and adherence. Thus, we need new treatments. The goal here was to strengthen networks that regulate TMSC through our individualized fMRI AI-NeuroModulation, termed iNM fMRI measures the magnitude and spatial extent of the ratio of oxygenated (O2) to deoxygenated hemoglobin (Hb). Our non-invasive, precision-medicine AI-iNM intervention: (1) target primary motor and sensory areas in the brain that regulate swallowing with 1mm level precision, (2) targets each patient's unique anatomical motor and sensory extents , and (3) iNM is and are guided by reinforcing or inhibiting the HbO2 intensity and extent of each patient's unique brain network, as opposed to self-regulation of the HbO2 intensity. Methods: Thirty healthy subjects participated in a 2-day study which included iNM and control conditions. We first computed the individualized TMSC cortical selectivity to be targeted in the iNM condition. Support vector machine (SVM) classified cortical tongue direction and tongue-at-rest patterns generated via iNM and control. We quantified the BOLD magnitude for each network by computing the area under the curve (AUC), variance and association between brain states and TMSC responses via dynamic causal modeling (DCM). Results: The mechanisms associated with attention-memory and sensorimotor iNM are: 1. 45% increase in the AUC's BOLD magnitude (p<0.001); 2. 14% decrease in the BOLD's intensity variance (p<0.01); and 3. 20% increase in network expansion (p<0.001). DCM uncovered 97% of the trials as pure motor signal, while 3% were noise in the iNM condition (compared to 83% of the TMSC trials and 17% noise in the control condition): 1. 70% motor-to-motor (M1, motor cerebellum, basal ganglia) and 70% motor-to-sensory; 2. 69% sensory-to-sensory (intraparietal lobule, insula, claustrum, sensory cerebellum, ACC); and 3. 70% sensorimotor-to-attention-memory network connectivities. Conclusion: iNM showed spatiotemporal causality between networks that regulate motor and sensory control, which can serve as clinical biomarkers for the RICN alleviation in head and neck cancer survivors.