Multivalent Lipid MVL5 Micellar Nanoparticles Exhibit Dramatically Increased Loading of Paclitaxel with PEGylation Enhancing Human Cancer Cell Penetration Depth and Cytotoxicity
Multivalent Lipid MVL5 Micellar Nanoparticles Exhibit Dramatically Increased Loading of Paclitaxel with PEGylation Enhancing Human Cancer Cell Penetration Depth and Cytotoxicity
Fisher, W. S.; Ghasemizadeh, A.; Roshan, S.; Goldstein, A.; Douglas, J.; Perez, R.; Li, Y.; Ewert, K. K.; Safinya, C. R.
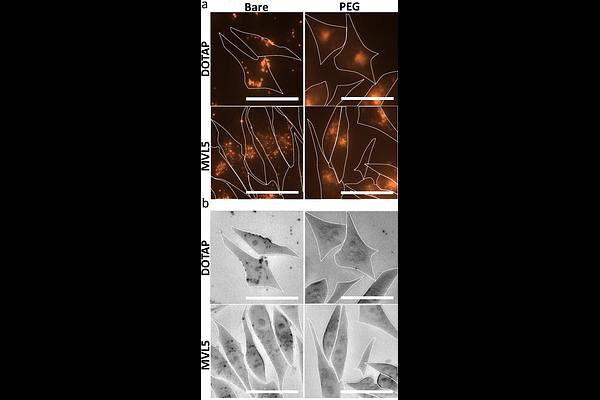
AbstractCationic liposomes (CLs) with chain-melted fluid membranes are promising nanocarriers of hydrophobic drugs in cancer chemotherapy, including the frequently employed drug paclitaxel (PTX). CL formulations containing univalent N-[2,3-dioleoyloxy-1-propyl]trimethylammonium chloride (DOTAP), like EndoTAG-1, have shown limited success in clinical trials and challenges like endosomal entrapment, limited PTX membrane solubility, and difficulty with in vivo tumor targeting remain. Incorporation of 10 mol% cone-shaped poly(ethylene glycol)-lipid (PEG-lipid) to DOTAP containing CLs transitions a fraction of the particles to micellar nanodiscs. These PTX-loaded PEGylated CLs and nanodiscs show enhanced cellular uptake in vitro and improved tumor penetration and proapoptotic activity compared to bare CLs in an in vivo solid breast cancer tumor model. Formulations incorporating the multivalent cationic lipid MVL5 (+5e) at 50 mol% form nanoparticles (NPs) comprised almost entirely of nanodiscs, and transition at 75 mol% MVL5 to short micellar rods coexisting with spheres, with rods further transitioning to long flexible rods upon PEGylation. Here, we report on the finding that MVL5-based micellar NPs with disc, rod, and spherical morphologies dramatically improve the solubility of PTX in their fluid lipid membranes by nearly three-fold compared to reference CLs modeled on the EndoTAG-1 formulation. Cell viability assays revealed that this improved PTX solubility for MVL5 micellar NPs leads to improved cytotoxic efficacy, which is further improved by PEGylation. Remarkably, using fluorescent microscopy and image particle analysis, we find that the cellular uptake and penetration depth of MVL5 nanoparticles is significantly improved by PEGylation. The findings are consistent with a model where the rate-limiting step of PTX delivery by cationic lipid NPs is diffusion of endocytic vesicles containing NPs through the actin mesh near the cell surface combined with the hoping rate of PTX from endosomal membrane to nearby microtubules. PEGylated cationic lipid nanoparticles containing MVL5 therefore represent a very promising hydrophobic cancer drug delivery vehicle for nanomedicine applications.