Bone Mineralization and Metabolism are Altered in a Rat Model of Brachial Plexus Birth Injury
Bone Mineralization and Metabolism are Altered in a Rat Model of Brachial Plexus Birth Injury
Fawcett, E. B.; Potts, J. R.; Dixit, N. N.; Saul, K. R.; Cole, J. H.
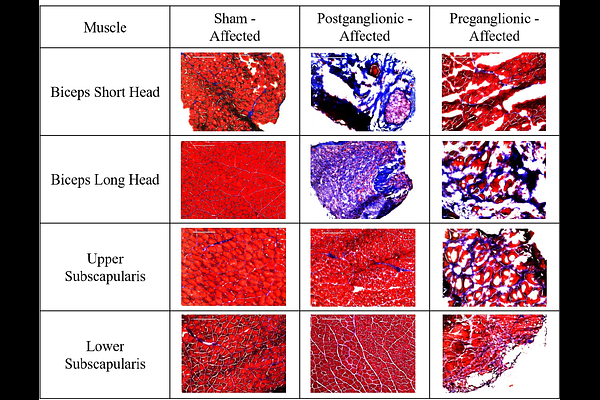
AbstractBrachial plexus birth injury (BPBI) is a common nerve injury incurred during a difficult childbirth when the brachial plexus nerve bundle is excessively stretched, resulting in functional arm impairment in 30-40% of those affected. Injury can present in two different locations, modeled in rats as postganglionic and preganglionic neurectomies. Osseous deformities are present following both injury types. However, the underlying factors behind these deformities are not fully understood. While past studies have explored muscle structure and altered mechanical joint loading as factors, bone metabolism, muscle composition, and muscle-bone crosstalk have not been fully explored. Using postganglionic and preganglionic BPBI rat models and a disuse model, bone metabolism, muscle composition, and muscle-bone crosstalk were explored. Dynamic histomorphometry and similar methods were used to characterize humeral growth and humeral growth plate activity to understand bone metabolism, muscle fibrosis was analyzed to assess muscle composition, and FGF-2 quantification was performed to assess muscle-bone crosstalk. Postganglionic injury portrayed more changes in the humeral diaphyseal region than preganglionic and displayed reduced bone metabolism on the endosteal surface while preganglionic displayed reduced bone metabolism on the periosteal surface. However, only preganglionic showed significantly lower growth plate activity. In regards to fibrosis, both injury types showed fibrosis in the biceps but only preganglionic showed fibrosis in the subscapularis. The limb disuse model did not show fibrosis. Additionally, preganglionic had an increased production of FGF-2 signaling more so in the subscapularis. Overall, deformities from postganglionic injury may be from bone formation and bone resorption while deformities from preganglionic injury are likely from an overall reduction in bone growth that is not solely from limb disuse. The fibrosis and FGF-2 signaling alterations seen are not likely to be the direct cause of osseous deformity and the drivers behind the alterations are likely different between postganglionic and preganglionic injuries.