Arsenic-sensing domain controls ACR3 transporter trafficking and function in Marchantia polymorpha
Arsenic-sensing domain controls ACR3 transporter trafficking and function in Marchantia polymorpha
Mizio, K.; Bonter, I.; Zbieralski, K.; Dolzblasz, A.; Tomaszewska, P.; Staszewski, J.; Wawrzycka, D.; Reymer, A.; Bialek, W.; Kriechbaumer, V.; Haseloff, J.; Wysocki, R.; Maciaszczyk-Dziubinska, E.
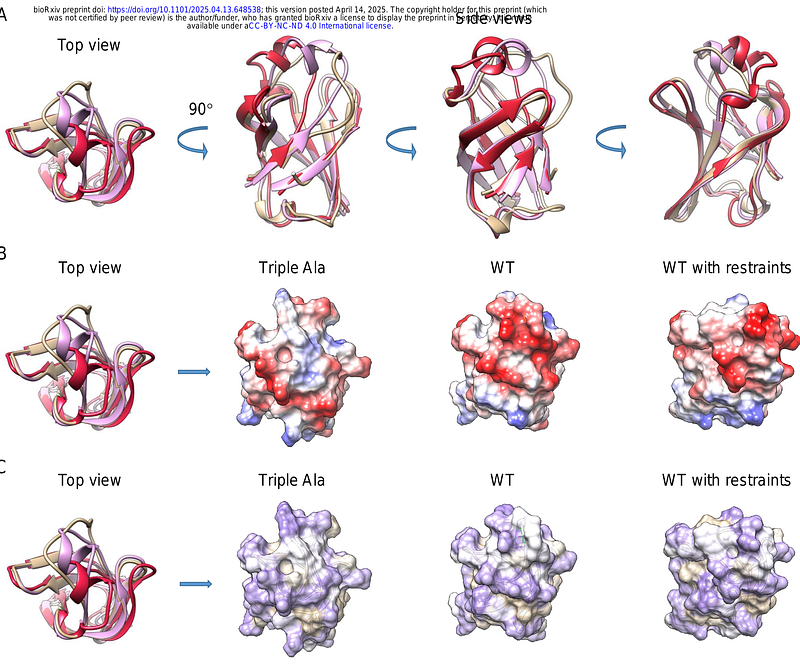
AbstractArsenic, a toxic and carcinogenic metalloid, is a pervasive environmental contaminant that threatens human health through contaminated water and food. The efflux of As(III) via ACR3 transporters is an ancient detoxification mechanism conserved across prokaryotes, fungi, and plants, with the notable exception of angiosperms. Despite their evolutionary significance, plant ACR3s remain largely uncharacterized. Here, we demonstrate that MpACR3, the ACR3 orthologue from the liverwort Marchantia polymorpha, functions as a metalloid/proton antiporter, conferring resistance to arsenicals and moderate tolerance to antimony. Additionally, we uncover an arsenic-sensing domain within MpACR3 that regulates its intracellular trafficking. Under normal conditions, MpACR3 sorting to the plasma membrane is delayed, resulting in its retention within Golgi bodies. However, As(III) binding to three cysteine residues in the N-terminal cytosolic domain induces a conformational change that facilitates MpACR3 trafficking to the plasma membrane. Furthermore, mutational analysis of a conserved arginine-based motif reveals that the N-terminal domain not only controls MpACR3 accumulation at the plasma membrane but also modulates its transport activity. Importantly, this arsenic-sensing domain is conserved among plant ACR3 transporters, suggesting a plant-specific adaptation to arsenic toxicity.