High-Purity Production of Endothelial Cells from Human Pluripotent Stem Cells
High-Purity Production of Endothelial Cells from Human Pluripotent Stem Cells
Yoshimoto, K.; Terada, S.; Kamei, K.-i.
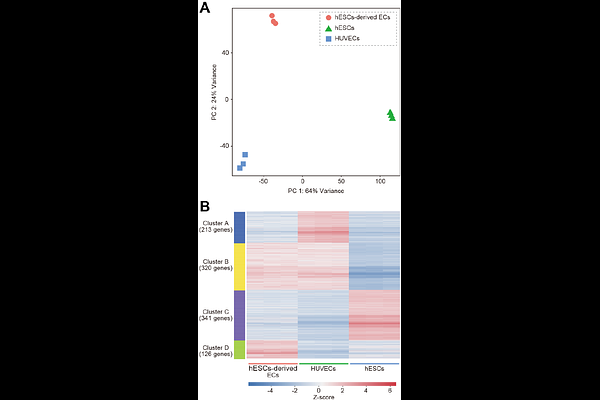
AbstractEndothelial cells (ECs) are essential for vascular network formation and tissue homeostasis, yet the fields of tissue engineering and vascularized organoid generation still relies heavily on human umbilical vein ECs (HUVECs), which are venous, allogeneic, and difficult to mature fully. Human pluripotent stem cells (hPSCs) offer an autologous, developmentally flexible alternative, but most differentiation protocols require fluorescence-activated cell sorting, limiting scalability. Here we present a streamlined method that produces highly pure ECs directly from human embryonic stem cells (hESCs) without cell sorting. Extending Wnt activation with CHIR99021 to three days maximizes mesoderm induction, and brief Notch blockade with DAPT during specification suppresses smooth-muscle commitment. The result is over 90 % CD31 CD144 ECs that display classic cobblestone morphology, robust DiI-acetylated LDL uptake, and capillary-like sprouting comparable to HUVECs. Bulk RNA barcoding and sequencing segregates the hESC-derived ECs from both HUVECs and undifferentiated hESCs and uncovers an arterial-skewed transcriptome: NOTCH1, DLL4, and CXCR4 are up-regulated, whereas venous markers (EPHB4, NRP2) are reduced. Enrichment of Notch-responsive pathways further supports an arterial-like identity. Although several adult functional genes (e.g., vWF, NOS3) are expressed at lower levels than in HUVECs, the protocol delivers a scalable source of developmentally relevant ECs ideal for vascularizing organoids derived from the same hPSCs and for future applications in drug screening, disease modeling, and cell-based vascular therapies.