Pathological Mechanisms of Motor Dysfunction in Familial Danish Dementia: Insights from a Knock-In Rat Model
Pathological Mechanisms of Motor Dysfunction in Familial Danish Dementia: Insights from a Knock-In Rat Model
Choudhury, A.; Yesiltepe, M.; Lashley, T.; Singh, V.; Yin, T.; Fernandez, A.; D'Adamio, L.; Ahn, H. J.
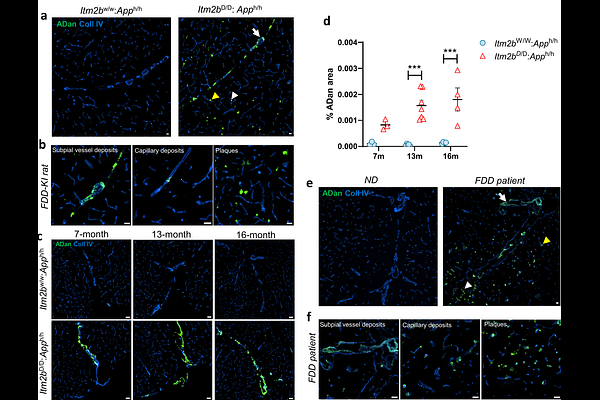
AbstractFamilial Danish Dementia (FDD) is a rare autosomal dominant neurodegenerative disorder caused by a mutation in the integral membrane protein 2B (ITM2b) gene. Clinically, FDD is characterized by cerebral amyloid angiopathy (CAA), cerebellar ataxia, and dementia. Notably, FDD shares several neuropathological features with Alzheimer\'s disease (AD), including CAA, neuroinflammation, and neurofibrillary tangles. In this study, we investigate the pathological mechanisms linking CAA, white matter damage, and motor dysfunction using a recently developed FDD knock-in (FDD-KI) rat model. This model harbors the Danish mutation in the endogenous rat Itm2b gene, along with an App gene encoding humanized amyloid-{beta} (A{beta}). Our analysis revealed substantial vascular Danish amyloid (ADan) deposition in the cerebellar subpial and leptomeningeal vessels of FDD-KI rats, showing an age-related increase comparable to that observed in human FDD patients. Additionally, vascular A{beta} deposits (A{beta}-CAA) were present in FDD-KI rats, but A{beta}-CAA patterns showed some differences between species. Motor function assessments in FDD-KI rats demonstrated age-accelerated motor deficits and gait abnormalities, mirroring the clinical characteristics of FDD patients. To further explore the mechanisms underlying these deficits, we examined cerebellar pathology and found age-related myelin disruption and axonal fiber loss, consistent with postmortem human FDD pathology. Cerebellar demyelination appeared to be driven by neuroinflammation, marked by increased microglial/macrophage activation in response to vascular amyloid deposition. Additionally, we observed extravascular fibrinogen leakage, indicating widespread vascular permeability in both white and gray matter, with fibrinogen deposits surrounding amyloid-positive vessels in aged FDD-KI rats and postmortem FDD cerebellum. These findings suggest that this FDD-KI rat model is the first animal model to recapitulate key neuropathological features of human FDD patients, including both ADan- and A{beta}-type CAA, neuroinflammation, and white matter lesions - pathologies that may underlie the motor and gait impairments seen in the disease.