Tissue-specific improvements in CD4+ T cell responses after treatment for visceral leishmaniasis.
Tissue-specific improvements in CD4+ T cell responses after treatment for visceral leishmaniasis.
Engel, J. A.; Rivera, F.; Crawford, B.; Lee, H. J.; Na, J.; Chang, K.; Gartlan, K.; Bukali, L.; Frame, T.; Wang, Y.; Haque, A.; Engwerda, C.; Engwerda, C.
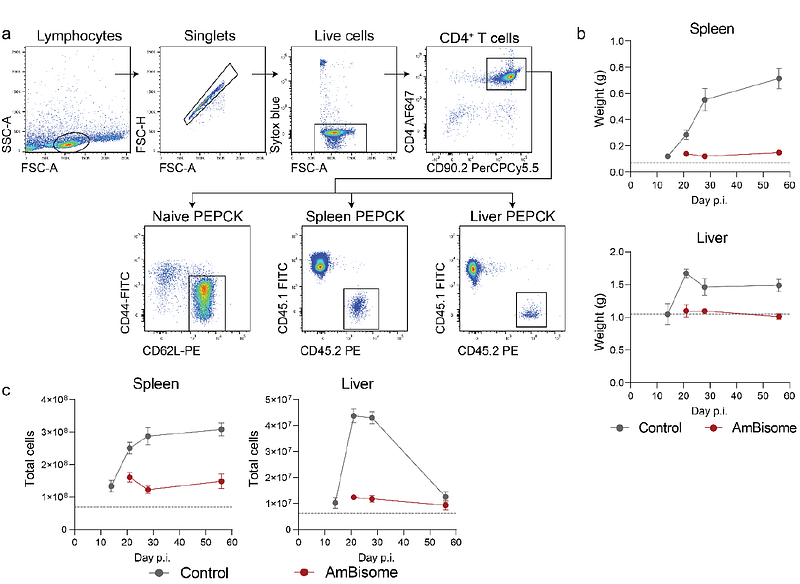
AbstractVisceral leishmaniasis (VL) is a life-threatening parasitic disease that requires robust CD4+ T cell-mediated immunity for parasite control. However, the heterogeneity and transcriptional dynamics of CD4+ T cell responses in VL remain poorly defined. In this study, we use a model of experimental VL with tissue-specific immunity and single-cell RNA sequencing to provide a high-resolution assessment of CD4+ T cell responses. Our analysis reveals the complexity of CD4+ T cell differentiation in VL, identifying distinct Th1 subsets with transcriptional heterogeneity that may reflect functional specialisation. Despite minimal transcriptional differences between splenic and hepatic CD4+ T cells, we identified shifts in subset composition, including the emergence of a stem-like CD4+ T cell population in the spleen, which was suppressed by the transcription factor Bhlhe40. Bhlhe40 deficiency skewed CD4+ T cell differentiation, impairing Th1 responses while promoting Tr1 cells, resulting in defective parasite control in the liver. Additionally, AmBisome treatment induced a profound transcriptional shift in CD4+ T cells, leading to the maintenance of stem-like CD4+ T cells in the spleen and the expansion of tissue resident memory-like cells in the liver. These findings uncover key regulatory mechanisms that shape CD4+ T cell differentiation in VL and provide insights into how immune-modulatory strategies could enhance long-term immunity.