Generation of a Three-dimensional Human Neurovascular Unit Model in a Microfluidic Chip
Generation of a Three-dimensional Human Neurovascular Unit Model in a Microfluidic Chip
Qiu, B.; Pompe, S.; Xenaki, K.; van Bergen en Henegouwen, P. M. P.; Oliveira, S.; Mastrobattista, E.; Caiazzo, M.
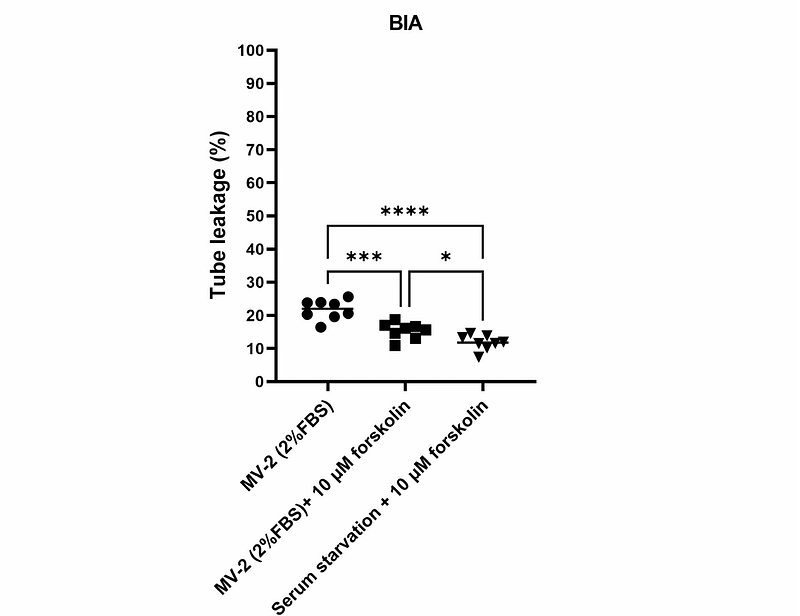
AbstractBackground The well-functioning of the neurovascular unit (NVU) is supported by the 3D brain physiological microenvironment that allows for extensive neural-neural and neural-vascular interactions. This microenvironment is normally hard to create in traditional in-vitro models such as the transwell model. Organ-on-a-chip (OOC) emerges as advanced model systems by providing better physiological microenvironments. However, NVU modeling in many chip platforms has not met a full 3D condition for neural cultures. Methods Here, we describe a novel NVU model generated in a microfluidic chip that reproduces the neural-neural and neural-vascular interactions in a full-3D format. The model features an extracellular matrix (ECM) environment that supports both a perfused brain endothelial vessel and 3D cultured neural cells (astrocytes and neurons) beside the tube. Culture conditions were comprehensively optimized for better endothelial tube integrity as well as ECM gel longevity. The model was used to model neuroinflammation-induced brain tube disruption and immune cell extravasation. Furthermore, as a drug testing platform, the model was explored for brain endothelial transcytosis of the heparin-binding EGF-like growth factor (HB-EGF) targeted nanobodies and the data was compared to a parallel transwell model. Results Immunofluorescent staining confirmed the expression of endothelial junctional proteins, as well as astrocytic and neuronal markers. The perfused brain endothelial tube exhibited resistance to paracellular leakage of 20 kDa FITC-dextran. Astrocytes and neurons growing in ECM gel developed extensive neural network and showed spontaneous neuronal firing. The neural-vascular interactions were formed through astrocyte migration and axonal outgrowth in the ECM gel towards the tube. Exposure to neuroinflammatory cytokines disrupted the tube barrier, resulting in increased barrier leakage and the recruitment of peripheral blood mononuclear cells (PBMCs) as well as their extravasation. Owing to full-3D model design, endothelial transcytosis and abluminal distribution of the fluorescently labeled HB-EGF targeting Nbs can be clearly visualized in situ. Compared to a transwell model counterpart, the NVU chip model performed better in revealing the binding and transcytosis specificity of the targeted nanobodies. Conclusions We demonstrate improved physiological relevance in this full-3D NVU-on-a-chip model. The model could become a faithful platform for NVU research under both healthy and diseased conditions, and can be used as a reliable drug testing platform that aims at developing novel brain-targeted therapeutics.