Computational modeling of necrosis in neural organoids
Computational modeling of necrosis in neural organoids
Pantula, A.; Zhou, B.; Morales Pantoja, I. E.; Fedotova, A.; George, D.; Alam El Din, D.-M.; Lysinger, A.; Smirnova, L.; Gracias, D. H.
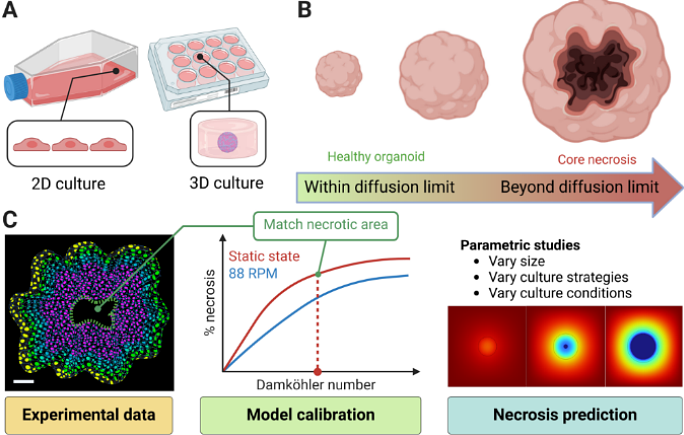
AbstractNeural organoids (NOs), also known as brain organoids, are derived from human-induced pluripotent stem cells and are Microphysiological Systems (MPS) of the brain that can recapitulate key aspects of neurodevelopment. They enable in vitro studies of brain development and disease mechanisms, providing disease models for various neurodegenerative or neurodevelopmental / degenerative disorders like Alzheimer\'s disease, microcephaly, and autism. There are many protocols to generate NOs with different complexities and sizes, varying from 400 microns to several mm in diameter, with a starvation-induced necrotic core eventually forming depending on the diameter and culture conditions. Thus, they can benefit from vascularization and more optimal culture conditions. There have been several attempts to decrease necrosis while growing larger NOs, such as by using orbital shaking or 2D/3D microfluidic chips, but only with limited success. In this study, we describe a 3D finite element model to simulate O2 starvation-induced necrosis in NOs using the Damkohler Number (Da) and the Michaelis-Menten kinetics. We measured the necrotic areas in NOs using fluorescent imaging and used them to calibrate the model with a specific Da. Using these calibrated values, we systematically compared simulations of different NO culture methods: static, orbital shaking, and microfluidic flow around organoids--highlighting their relative impacts on nutrient diffusion and necrosis. We observed that these culture strategies cannot prevent necrosis beyond a diameter of ~800 microns. Based on these findings, we propose that 3D spatial perfusion, achieved through uniformly distributed fluidic capillaries within the NO, could significantly reduce necrosis. We conducted parametric studies on capillary spacing, density, and layout. Our calibrated model offers insights for designing next-generation microfabricated bioreactors and culture devices, not just for NOs but also for all 3D tissue engineering and organoid research.