Small tau aggregates exhibit disease-specific molecular profiles across tauopathies
Small tau aggregates exhibit disease-specific molecular profiles across tauopathies
Böken, D.; Huang, M.; Wu, Y.; Fertan, E.; Lam, J. Y. L.; Meisl, G.; Baig, S.; Rowe, J.; Smith, C.; Quaegebeur, A.; Cox, D.; McEwan, W. A.; Klenerman, D.
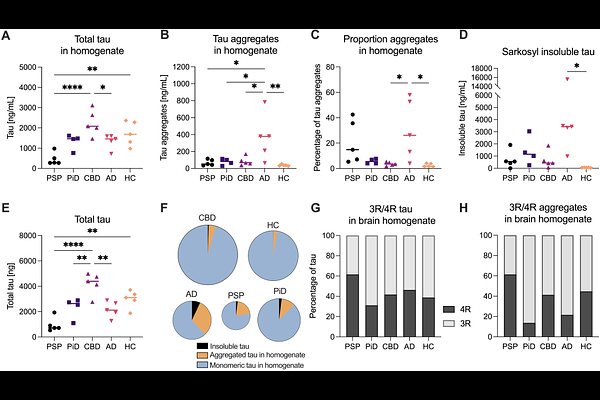
AbstractTauopathies are neurodegenerative diseases marked by pathological tau aggregation. While disease-specific folds of insoluble tau filaments have been established, it remains unclear whether the smaller, earlier species also differ across tauopathies. Here, we characterise these small tau aggregates from post-mortem brain of individuals with Alzheimer\'s disease (AD), progressive supranuclear palsy (PSP), corticobasal degeneration, Pick\'s disease, and healthy controls. Using two complementary single-molecule assays, we confirm that small tau aggregates vary in abundance, morphology, and post-translational modifications. AD features specific long, fibrillar-shaped aggregates enriched in phospho-epitopes, while PSP aggregates are shorter, round, and selectively phosphorylated at serine-356, a site we identify as correlating with markers of inflammation and apoptosis. Aggregate properties co-vary with cellular stress signatures and align with disease-specific seeding profiles, suggesting distinct pathological mechanisms. These findings suggest that small tau aggregates are not a shared intermediate, but instead encode disease-specific mechanisms, with potential as both biomarkers and therapeutic targets.