Molecular dynamics unveils multiple-site binding of inhibitors with reduced activity on the surface of dihydrofolate reductase
Molecular dynamics unveils multiple-site binding of inhibitors with reduced activity on the surface of dihydrofolate reductase
Araki, M.; Ekimoto, T.; Takemura, K.; Matsumoto, S.; Tamura, Y.; Kokubo, H.; Bekker, G.-J.; Yamane, T.; Isaka, Y.; Sagae, Y.; Kamiya, N.; Ikeguchi, M.; Okuno, Y.
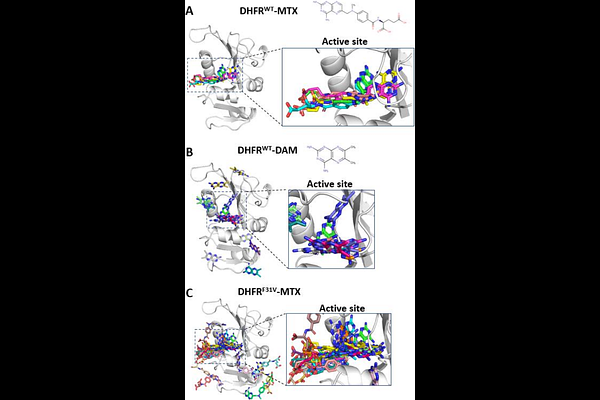
AbstractThe sensitivity to protein inhibitors is altered by modifications or protein mutations, as represented by drug resistance. The mode of stable drug binding to the protein pocket has been experimentally clarified. However, the nature of the binding of inhibitors with reduced sensitivity remains unclear at the atomic level. In this study, we analyzed the thermodynamics and kinetics of inhibi-tor binding to the surface of wild-type and mutant dihydrofolate reductase (DHFR) using molecular dynamics simulations com-bined with Markov state modeling. A strong inhibitor of methotrexate (MTX) showed a preference for the active site of wild-type DHFR with minimal binding to unrelated (secondary) sites. Deletion of a side-chain fragment in MTX largely destabilized the active site-bound state, with clear evidence of binding to secondary sites. Similarly, the F31V mutation in DHFR diminished the specificity of MTX binding to the active site. These results reveal the presence of multiple-bound states whose stabilities are com-parable to or higher than those of the unbound state, suggesting that a reduction in the binding affinity for the active site signifi-cantly elevates the fractions of these states. This study sheds light on the specific drug recognition by proteins and the selectivity of drug binding sites on protein surfaces.