Hydrolysis-dependent severing tunes internal monomeric heterogeneity to shape actin length distributions
Hydrolysis-dependent severing tunes internal monomeric heterogeneity to shape actin length distributions
Ray, S.; Banerjee, B.; Mohapatra, L.; Das, D.
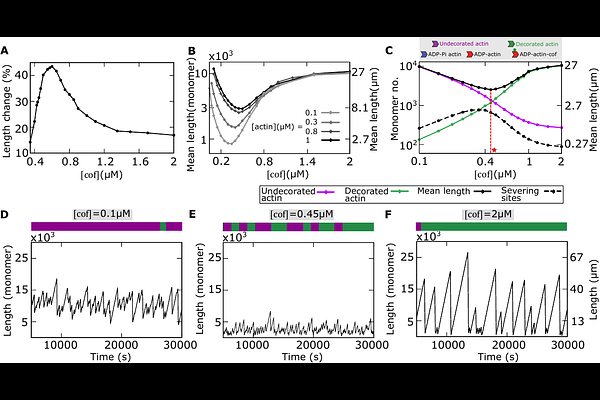
AbstractThe coordination of actin-binding proteins (ABPs) is essential for forming a wide range of actin structures that drive vital cellular processes. Many of the ABPs, like the severing protein cofilins, exhibit affinities dependent on the nucleotide state of actin monomers. Experiments have shown that cofilins interact with actin filaments cooperatively in a concentration-dependent manner, where it severs less at both low and high concentrations. However, how this nucleotide state-dependent severing influences actin filament distributions is poorly understood. Here, we propose a computational model of actin filament dynamics that combines stochastic polymerization, phosphate release, hydrolysis-dependent cooperative binding of cofilins, and severing of actin monomers. This model simultaneously reproduces key experimentally observed features: The non-monotonic change of the filament length and the monotonic decay in actin cap size with the increasing cofilin concentration, as well as the linear increase of the cap size with the actin concentration. We elucidate how both these emerge from the tuning of the heterogeneity in the arrangement of cofilin decorated and undecorated monomers within a filament. We also predict that, with varying cofilin concentrations, steady-state length distributions can change from symmetric bell-shaped to long-tailed skewe d shapes. We show that, in some regimes, our model simplifies to a coarse-grained model enabling us to mathematically predict that the distribution of undecorated monomers is exponential with a decay exponent that depends on cofilin and actin concentration. This study highlights the importance of non equilibrium processes that can modulate structural heterogeneity within a filament to shape actin length variation.