Fusion transcription factor dosage controls cell state in rhabdomyosarcoma
Fusion transcription factor dosage controls cell state in rhabdomyosarcoma
Hoffman, R. A.; Wang, M.; Sunkel, B. D.; Nguyen, T. H.; Lopez-Nava, J.; Chatterjee, B.; Sun, W.; Barr, F. G.; Stanton, B. Z.
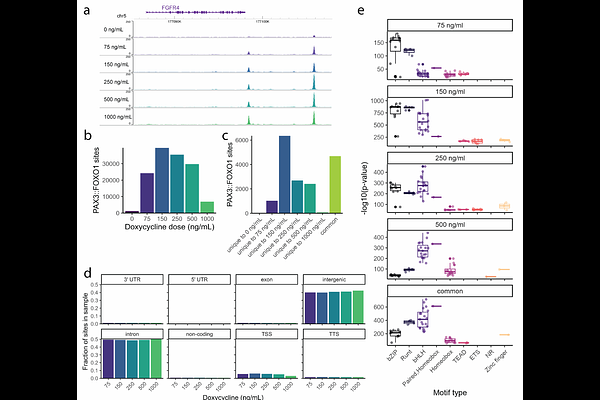
AbstractIn the fusion-positive subset of rhabdomyosarcoma, the PAX3::FOXO1 oncoprotein is the most common fusion driver. We previously established a human myoblast system for inducible expression of PAX3::FOXO1. In the current study, we modulate PAX3::FOXO1 protein expression to understand the epigenetic and phenotypic functions at different PAX3::FOXO1 levels. Proliferative and oncogenic outcomes depend on PAX3::FOXO1 dosage in this system with transformation dominant at intermediate levels and growth suppression dominant at high levels. After prolonged PAX3::FOXO1 expression, there is dosage-dependent heterogeneity in single cell gene expression profiles. We observe a dosage-specific effect for PAX3::FOXO1 chromatin recognition and identify factors that modulate PAX3::FOXO1 chromatin binding. PAX3::FOXO1 dosage affects expression signatures related to cell cycle, epithelial-mesenchymal transition, and myogenesis. Whereas intermediate PAX3::FOXO1 expression maximizes chromatin binding to modulate gene expression, high PAX3::FOXO1 expression alters S phase progression and increases accessibility behind the replication fork. We conclude that PAX3::FOXO1 exerts dosage-dependent functions to influence epigenetic heterogeneity in fusion-positive rhabdomyosarcoma.