Secreted antigen A peptidoglycan hydrolase is essential for Enterococcus faecium cell separation and priming of immune checkpoint inhibitor cancer therapy
Secreted antigen A peptidoglycan hydrolase is essential for Enterococcus faecium cell separation and priming of immune checkpoint inhibitor cancer therapy
Klupt, S.; Fam, K. T.; Zhang, X.; Chodisetti, P. K.; Mehmood, A.; Boyd, T.; Grotjahn, D.; Park, D.; Hang, H. C.
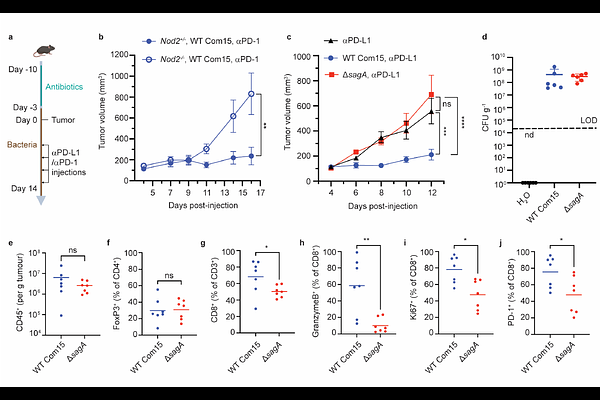
AbstractEnterococcus faecium is a microbiota species in humans that can modulate host immunity, but has also acquired antibiotic resistance and is a major cause of hospital-associated infections. Notably, diverse strains of E. faecium produce SagA, a highly conserved peptidoglycan hydrolase that is sufficient to promote intestinal immunity and immune checkpoint inhibitor antitumor activity. However, the essential functions of SagA in E. faecium were unknown. Here we report that deletion of sagA impaired E. faecium growth and resulted in bulged and clustered enterococci due to defective peptidoglycan cleavage and cell separation. Moreover, {Delta}sagA showed increased antibiotic sensitivity, yielded lower levels of active muropeptides, displayed reduced activation of the peptidoglycan pattern-recognition receptor NOD2, and failed to promote cancer immunotherapy. Importantly, plasmid-based expression of SagA, but not its catalytically-inactive mutant, restored {Delta}sagA growth, production of active muropeptides and NOD2 activation. SagA is therefore essential for E. faecium growth, stress resistance and activation of host immunity.