A reverse-transcription loop-mediated isothermal amplification (RT-LAMP) assay for the rapid colorimetric detection of pepper mild mottle virus (PMMoV)
A reverse-transcription loop-mediated isothermal amplification (RT-LAMP) assay for the rapid colorimetric detection of pepper mild mottle virus (PMMoV)
Gross, A. J.; Lopez, S.; Rogers, A.; Adkins, S.; Breitbart, M.
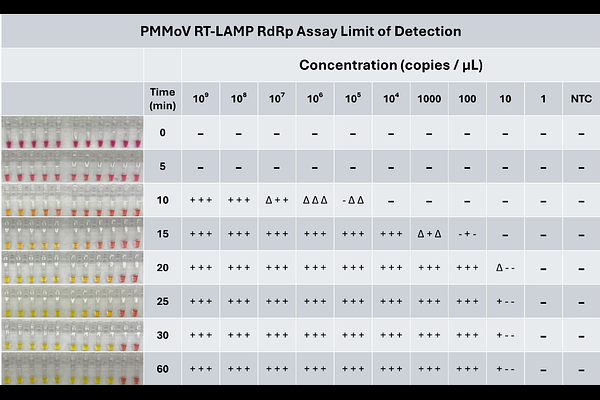
AbstractPepper mild mottle virus (Tobamovirus capsica, PMMoV) is a plant virus in the genus Tobamovirus that infects peppers and other members of the family Solanaceae. The virus is transmitted mechanically, poses a significant threat to crops globally, and is one of the most abundant viruses found in human feces and wastewater. Two colorimetric reverse-transcription loop-mediated isothermal amplification (RT-LAMP) assays were developed to detect PMMoV, one targeting the RNA-dependent RNA-polymerase (PMMoV_RdRp) and one targeting the coat protein (PMMoV_CP). Synthetic gBlock positive controls were used to determine the detection limit of each assay. PMMoV_RdRp detected PMMoV at concentrations greater than or equal to 100 copies/L, the same sensitivity as the RT-PCR assay for this gene. In contrast, the detection limit of the PMMoV_CP RT-LAMP assay was an order of magnitude greater. Both assays were specific to PMMoV and did not amplify plant host tissue or related tobamoviruses. Since these RT-LAMP assays do not require specialized laboratory equipment and yield positive results within 20-30 minutes, they are advantageous for point-of-use testing. Overall, the RT-LAMP assays described here are sensitive, specific, and more rapid than existing methods for PMMoV detection and quantification and thus have potential widespread applications for agriculture, wastewater treatment assessment, recreational water quality testing, and food safety.