Mechanisms controlling the plasma membrane targeting and the nanodomain organization of the plant SPFH protein HIR2
Mechanisms controlling the plasma membrane targeting and the nanodomain organization of the plant SPFH protein HIR2
Danek, M.; Hdedeh, O.; Boutet, J.; Safi, H.; Abuzeineh, A.; Martin Barranco, A.; Fiche, J.-B.; Mercier, C.; Nollmann, M.; BOUTTE, Y.; Santoni, V.; MONGRAND, S.; Martiniere, A.; Zelazny, E.
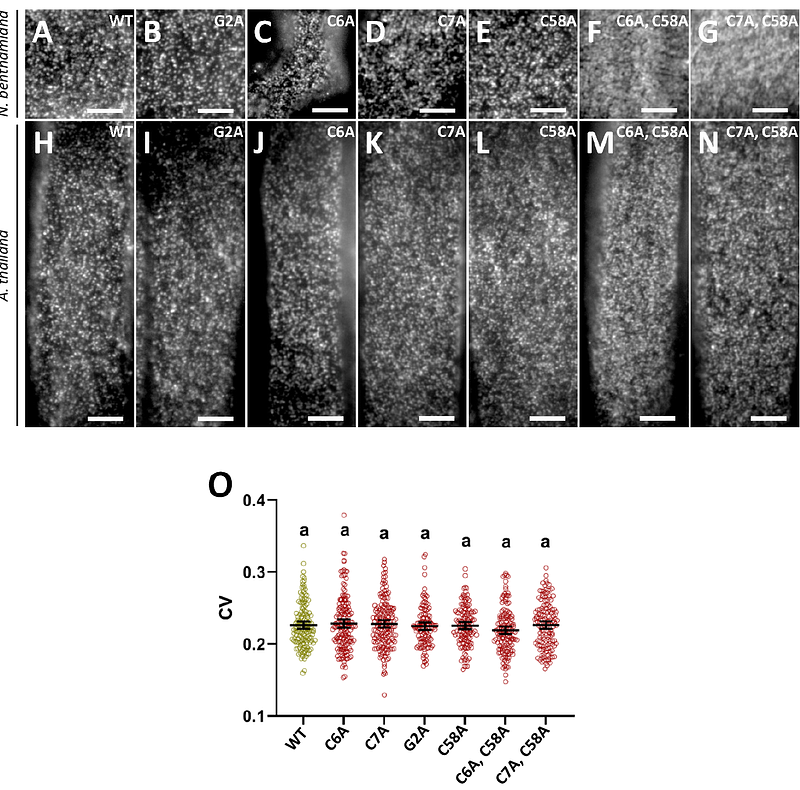
AbstractHIR2 is a plant-specific protein belonging to the superfamily of SPFH domain-containing proteins that were proposed to play scaffolding functions in membranes. HIR2 organizes in plasma membrane (PM) nanodomains that correspond to nanometric scale structures enriched in specific lipids and proteins acting as signaling/regulation hubs. So far, how PM nanodomains are formed and maintained in plant cells remains largely unknown. Combining state of the art microscopy techniques, we investigated the mechanisms governing the trafficking and the organization into nanodomains of Arabidopsis HIR2 protein. We revealed that the mono S-acylation of HIR2 either on C6 or on C7 was required for HIR2 targeting to the PM of Arabidopsis cells, independently of the conventional secretory pathway. Investigating the mechanisms implicated in the arrangement of HIR2 into nanodomains, we provided evidences that the lipid composition in sterols and very long chain fatty acids of the PM influenced HIR2 organization. HIR2 forms oligomers and we demonstrated here that the C-terminal part of HIR2 is required for self-assembly, similarly to animal SPFH proteins. Interestingly, we highlighted that the oligomerization of HIR2 is essential for its organization in nanodomains and to ensure HIR2 lateral stability in the PM. Overall, we propose that HIR2 nanodomain organization is a complex mechanism relying on different parameters including PM lipid composition and oligomerization. HIR proteins are involved in plant immunity. Here, we revealed that HIR2 nanodomain organization is required to boost the apoplastic ROS burst induced by the bacterial peptide flg22.