CslA and GlxA from Streptomyces lividans form a functional cellulose synthase complex
CslA and GlxA from Streptomyces lividans form a functional cellulose synthase complex
Zhong, X.; Nicolardi, S.; Ouyang, R.; Wuhrer, M.; Du, C.; van Wezel, G. P.; Vijgenboom, E.; Briegel, A.; Claessen, D.
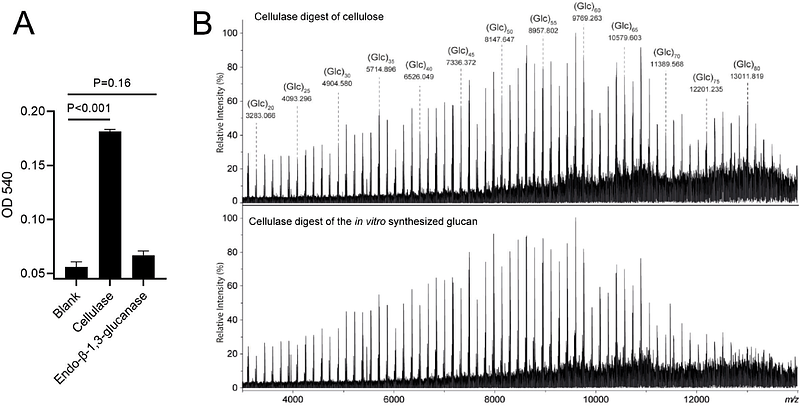
AbstractFilamentous growth of streptomycetes coincides with the synthesis and deposition of an uncharacterized protective glucan at hyphal tips. Synthesis of this glucan depends on the integral membrane protein CslA and the radical copper oxidase GlxA, which are part of a presumably large multiprotein complex operating at growing tips. Here, we show that CslA and GlxA interact by forming a protein complex that is sufficient to synthesize cellulose in vitro. Mass spectrometry analysis revealed that the purified complex produces cellulose chains with a degree of polymerization of at least 80 residues. Truncation analyses demonstrated that the removal of a significant extracellular segment of GlxA had no impact on complex formation, but significantly diminished activity of CslA. Altogether, our work demonstrates that CslA and GlxA form the active core of the cellulose synthase complex and provides molecular insights into a unique cellulose biosynthesis system that is conserved in streptomycetes.