Non-DNA-damaging DNA-PK activation improving hearing and prolonging life due to NAD+ and SIRT upregulation
Non-DNA-damaging DNA-PK activation improving hearing and prolonging life due to NAD+ and SIRT upregulation
Honkura, Y.; Suzuki, T.; Kujirai, R.; Kanno, S.; Matsumoto, Y.; Tanaka, Y.; Kowit, H.; Nagatoishi, S.; Tyshkovskiy, A.; Kasahara, T.; Tongu, Y.; Bohan, Z.; Kashiwagi, H.; Saegusa, C.; Fujioka, M.; Usami, R.; Chikuma, S.; Tokifuji, Y.; Matsuhashi, T.; Oikawa, Y.; Komatsu, H.; Murayama, K.; Sugasawa, T.; Nanto-Hara, F.; Taguchi, K.; Saigusa, D.; Suzuki, C.; Sato, T.; Suzuki, J.; Owada, Y.; Niizuma, K.; Endo, H.; Hashimoto, K.; Toyohara, T.; Tsumoto, K.; Anderson, P.; Gladyshev, V. N.; Hayashi, K.-i.; Katori, Y.; Tomioka, Y.; Abe, T.
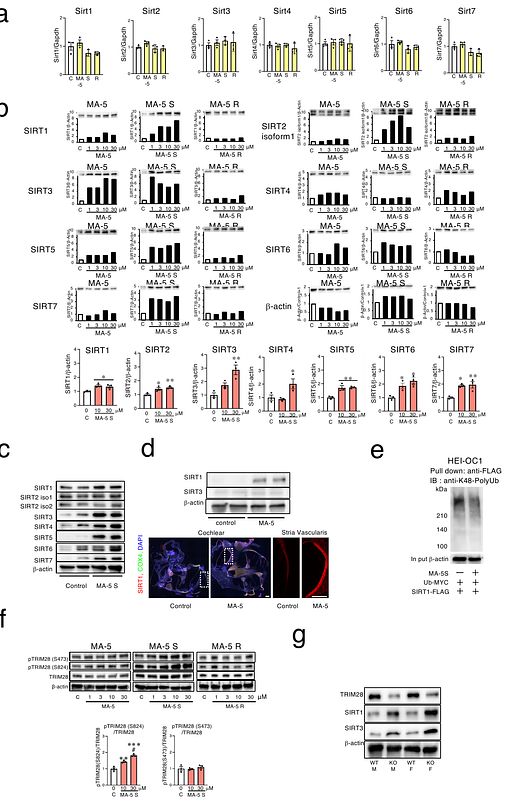
AbstractEmerging evidence strongly supports a close relationship between age-related hearing loss and frailty, highlighting the importance of early detection and intervention. Recently, we invented a mitochondria-homing drug named mitochonic acid 5 (MA-5), that increases the adenosine triphosphate (ATP) levels, rescue mitochondrial function, and protect tissue damages. Currently, the phase I clinical trial has been finished in Japan (jRCT2031210495) and the phase 2 clinical trial has already been approved by PMDA. Here we show that MA-5 improved various types of hearing loss in mouse models. Structural chemical bioanalysis revealed that MA-5 is a mixture of equal amount of S- and R- enantiomer and both S- and R-enantiomer increase ATP by binding mitochondrial protein, mitofilin. However, S-enantiomer significantly increased the NAD+ levels by binding to the NAD+-producing key enzyme nicotinamide phosphoribosyltransferase (NAMPT). Moreover, the S-enantiomer increased the sirtuin 1 protein by suppressing polyubiquitination induced by tripartite motif containing 28 (TRIM28) phosphorylation which was triggered by DNA-dependent protein kinase (DNA-PK) activation in the absence of DNA damage. Transcriptomic signatures showed that the signature of MA-5 shows an inverse correlation with aging and mortality and is oriented in the same direction as the OSKM-related iPSCs, suggesting the modification of aging pathways. Oral administration of MA-5 to mitochondrial disease model mouse showed increased survival. Our findings suggest that, in addition to enhancing ATP levels, the coordinated regulation of NAD metabolism, SIRT protein expression, and DNA-PK activity-constituting a novel therapeutic triad may contribute to the amelioration of hearing impairment and mitochondrial dysfunction, thereby improving life prognosis.