Physical confinement and phagocytic uptake induce directional migration
Physical confinement and phagocytic uptake induce directional migration
Paulson, S. G.; Liu, S.; Rotty, J. D.
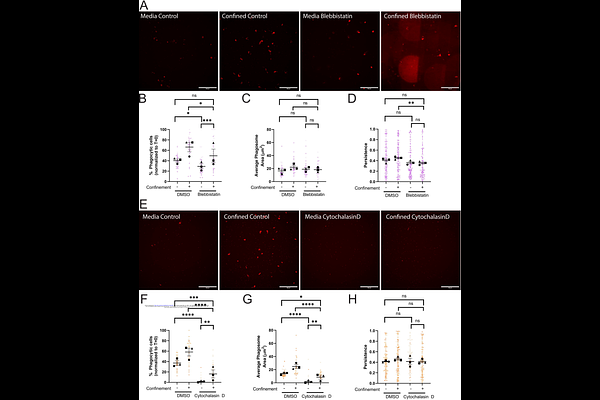
AbstractPhysical confinement is not routinely considered as a factor that influences phagocytosis, which is typically assayed in unconfined settings in vitro. BV-2 microglia-like cells were used to interrogate the impact of confinement on IgG-mediated phagocytosis side by side with unconfined cells. Confinement acted as a potent phagocytic driver, greatly increasing the fraction of phagocytic cells in the population compared to the unconfined setting. Arp2/3 complex and myosin II contributed to this effect. Remarkably, confinement partially rescued phagocytic uptake upon myosin II disruption. In addition, cells under confinement were partially resistant to the actin-depolymerizing drug cytochalasin D. Unexpectedly, we observed that bead uptake stimulated persistent migration, a process we term phagocytic priming. Integrin-dependent adhesion was required for phagocytic priming in unconfined and confined settings, but was dispensable for phagocytic uptake. The cytoskeletal requirements for phagocytic priming differed depending on confinement state. Myosin II and Arp2/3 complex were required for phagocytic priming under confinement, but not in unconfined settings. As with phagocytosis, cytoskeleton-dependent priming of motility shifts depending on physical confinement status. Phagocytic priming may facilitate innate immune function by driving cells to more efficiently surveil their local microenvironment in response to wounds or trauma.