Apremilast improves cardiomyocyte cohesion and arrhythmia in different models for arrhythmogenic cardiomyopathy
Apremilast improves cardiomyocyte cohesion and arrhythmia in different models for arrhythmogenic cardiomyopathy
Stangner, K.; Dervishi, O.; Kuhnert, J.; Wendt, C.; Pathak, S.; Shoykhet, M.; Olivares Florez, S.; Moztarzadeh, S.; Opsteen, J.; Suniasih Wohlfarth, N. L. C.; Biller, R.; Graf, E.; Westphal, D. S.; Williams, T.; Gerull, B.; Saric, T.; Yeruva, S.; Waschke, J.
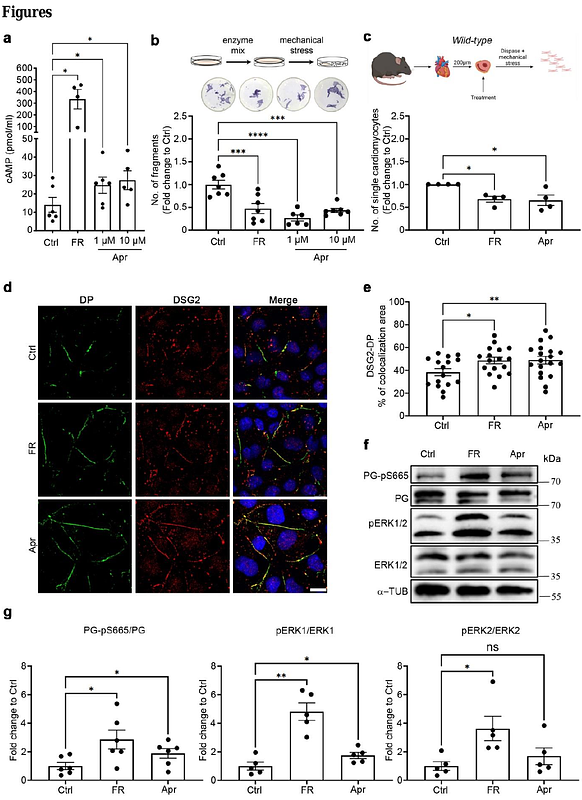
AbstractBackground: Arrhythmogenic cardiomyopathy (ACM) is a genetically inherited desmosome heart disease leading to life-threatening arrhythmias and sudden cardiac death. Currently, ACM treatment paradigms are merely symptom targeting. Recently, apremilast was shown to stabilize keratinocyte adhesion in the desmosomal disease pemphigus vulgaris. Therefore, this study investigated whether apremilast can be a therapeutic option for ACM. Methods: Human induced pluripotent stem cells from a healthy control (hiPSC) and an ACM index patient (ACM-hiPSC) carrying a heterozygous desmoplakin (DSP) gene mutation (c.2854G>T, p.Glu952Ter), confirmed by whole exome sequencing (WES), were established. Cyclic-AMP ELISA, dissociation assay, immunostaining, and Western blotting analyses were performed in human iPSC-derived cardiomyocytes (hiPSC-CMs), murine HL-1 cardiomyocytes, and cardiac slices derived from wild-type (WT) mice, plakoglobin (PG, Jup) knockout (Jup-/-) (murine ACM model) or PG Serine 665 phosphodeficient (JUP-S665A) mice. Microelectrode array (MEA) analyses in ventricular cardiac slices and Langendorff heart perfusion were performed to analyze heart rate variability and arrhythmia. Results: ACM-hiPSC derived cardiomyocytes (ACM-hiPSC-CMs) revealed a significant loss of cohesion, which was rescued by apremilast. Further, treatment with apremilast strengthened basal cardiomyocyte cohesion in HL-1 cells and WT murine cardiac slices, paralleled by phosphorylation of PG at Serine 665 in human and murine models. In HL-1 cells, apremilast in addition activated ERK1/2, inhibition of which abolished apremilast-enhanced cardiomyocyte cohesion. Further, dissociation assays in slice cultures from JUP-S665A and Jup-/- mice revealed that PG is crucial for apremilast-enhanced cardiomyocyte cohesion. In parallel to enhanced cell adhesion, MEA and Langendorff measurements from WT and Jup-/- mice demonstrated decreased heart rate variability and arrhythmia after apremilast treatment. Conclusions: Apremilast improves loss of cardiomyocyte cohesion, enhances localization of DSG2, and reduces arrhythmia in human and murine models of ACM ex vivo and in vitro, providing a novel treatment strategy for ACM by preserving desmosome function.