Microtubule curling as an efficient readout to uncover fundamental concepts of axonal cell biology
Microtubule curling as an efficient readout to uncover fundamental concepts of axonal cell biology
Voelzmann, A.; Owens, M.; Beaven, R.; Cairns, W.; Elliot, A.; Feng, S.-H.; Goncaves-Pimentel, C.; Hahn, I.; Jones, E.; Norris, K.; Murphy, T.; Liew, Y.-T.; Lorenzo-Cisneros, L.; Fuelle, J.; Pinho-Correia, L. M.; Qu, Y.; Sharma, T.; Sanchez-Soriano, N.; Prokop, A.
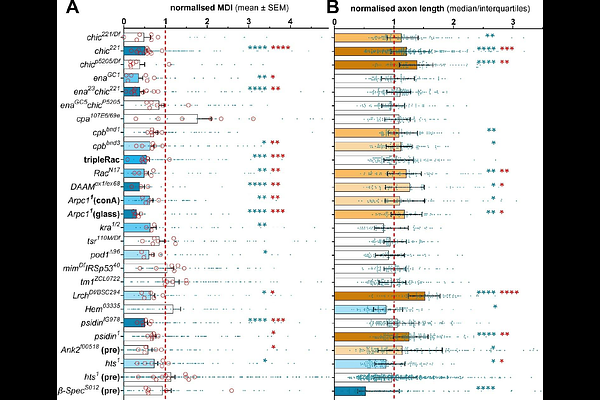
AbstractIn neurodegeneration, axons tend to be prime lesion sites. Axons are the slender, up-to-meter-long processes of nerve cells that essentially wire nervous systems. These delicate structures must survive for an organism\'s lifetime. Their long-term maintenance requires complex cell biology to be locally present in axons, and this depends on the steady supply with organelles and materials. These are delivered by motor protein-driven axonal transport, which uses continuous bundles of MTs as highways that run interrupted from the cell body to the axon tip. By studying the regulation of these bundles in our past work, we identified many conditions in which microtubules become severely disorganised, a phenotype expected to be detrimental for axons. Further work on these conditions led us to propose the Dependency Cycle of Local Axon Homeostasis model explaining how aberration of different cell biological processes can lead to one common outcome - as is the case in neurodegeneration. Here we put this model to the test. Through live imaging we trace the origins and time course of microtubule disorganisation in normal and mutant neurons, and our findings confirm key mechanisms proposed in our model. Combining published data with the re-analysis and new investigation of 105 gene deficiencies from a wide range of cell biological contexts, we find that ~40% cause the shared microtubule disorganisation phenotype, thus clearly corroborating a key idea of the cycle. Through probing the phenotypes of 18 genes for ROS involvement, we find them to correctly classify into a mechanical ROS-independent versus a physiological ROS-dependent group, providing strong support for a central aspect of the dependency model.