Piezo1 stretch-activated channel activity differs between bone marrow-derived and cardiac tissue-resident macrophages
Piezo1 stretch-activated channel activity differs between bone marrow-derived and cardiac tissue-resident macrophages
Simon-Chica, A.; Klesen, A.; Emig, R.; Chan, A.; Gruen, D.; Lother, A.; Hilgendorf, I.; Ravens, U.; Kohl, P.; Schneider-Warme, F.; Peyronnet, R.
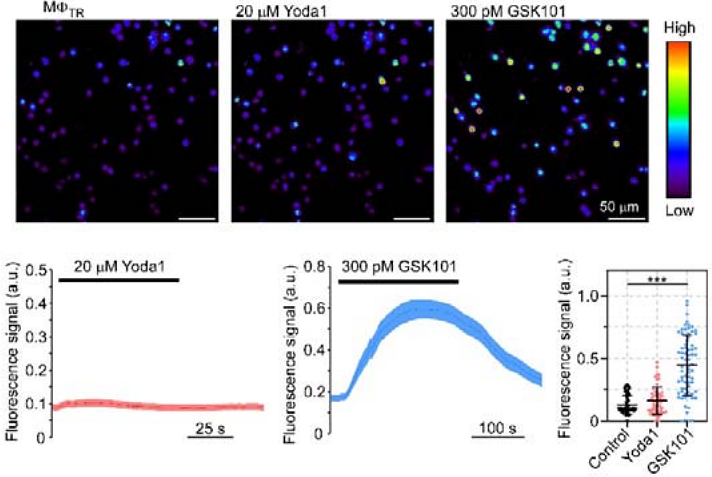
AbstractMacrophages (M{Phi}) play pivotal roles in tissue homeostasis and repair. Their mechanical environment recently emerged as a key modulator of various cell functions, and M{Phi} mechanosensitivity is likely to be critical for cellular activity in particular in a rhythmically contracting organ such as the heart. M{Phi}, in-vitro-differentiated from bone marrow (M{Phi}BM), form a popular cell model for research. This study explores the activity of stretch-activated ion channels (SAC) in murine M{Phi}BM and compares it to SAC activity in cardiac tissue-resident M{Phi} (M{Phi}TR). Our main findings are: i) M{Phi}BM and M{Phi}TR have stretch-induced currents, indicating expression of functional SAC at their plasma membrane; ii) the current profiles in M{Phi}BM and in M{Phi}TR show characteristics of cation non-selective SAC; iii) unlike in M{Phi}BM, Piezo1 ion channel activity at the plasma membrane of M{Phi}TR is not detectable, neither by assessing electrophysiological activity using the patch clamp technique, nor by measuring cytosolic calcium concentration upon perfusion with Yoda1, a Piezo1 channel agonist. In mature scars after ventricular cryoablation, stretch-induced current characteristics of M{Phi}TR are not significantly different compared to non-injured control tissue, even though scars are expected to contain a mix of pre-existing and circulation-recruited M{Phi}. This suggests that M{Phi} invading injured cardiac tissue either phenoconvert their mechanosensitivity from M{Phi}BM to M{Phi}TR, or that the in vitro differentiation protocols used to obtain M{Phi}BM generate cells that differ from M{Phi} recruited from the circulation during tissue repair in vivo. Further investigations will explore SAC identity in lineage-traced M{Phi} in scar tissue, and compare mechanosensitivity of circulating monocytes with that of M{Phi}BM.