Diurnal rhythm causes metabolic crises in the cyanobacterial mutants of c-di-AMP signalling cascade
Diurnal rhythm causes metabolic crises in the cyanobacterial mutants of c-di-AMP signalling cascade
Haffner, M.; Mantovani, O.; Spaet, P.; Macek, B.; Hagemann, M.; Forchhammer, K.; Selim, K. A.
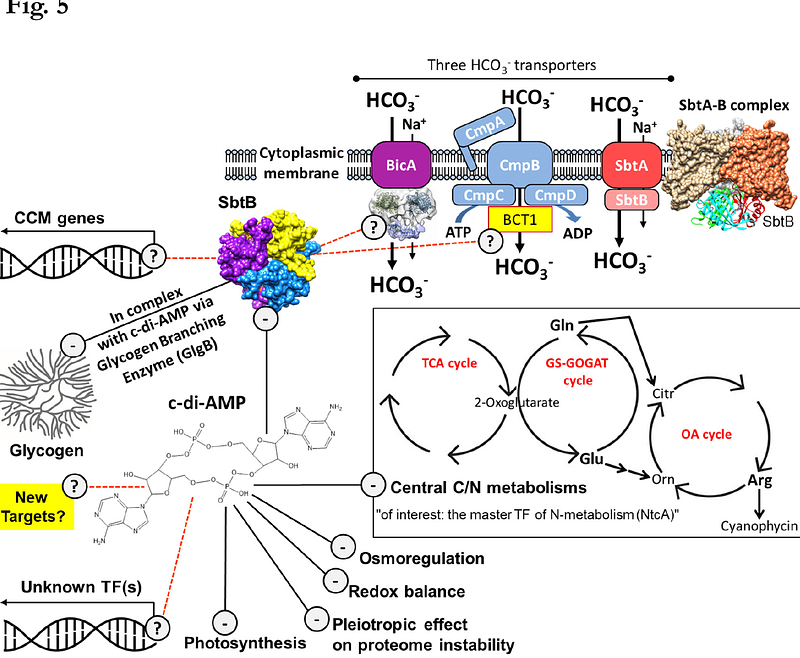
AbstractIn nature, the photoautotrophic lifestyle of cyanobacteria has to cope with the successive diurnal changes in light supply. Light supply throughout the day enables photosynthesis and glycogen biosynthesis, while night phases require the switch to a heterotrophic-like lifestyle relying on glycogen catabolism. We previously highlighted a unique function of the carbon control protein, SbtB, and its effector molecule c-di-AMP, for the nighttime survival of cyanobacteria through the regulation of glycogen anabolism. However, the extent to which c-di-AMP and SbtB impact the cellular metabolism for day-night survivability remained elusive. To gain better understanding of cellular processes regulated by SbtB or c-di-AMP, we compared the metabolomic and proteomic landscapes of {Delta}sbtB and the c-di-AMP-free ({Delta}dacA) mutants of the model strain Synechocystis sp. PCC 6803. While our results indicate that the cellular role of SbtB is restricted to carbon/glycogen metabolism, the diurnal lethality of {Delta}dacA seems to be a sum of dysregulation of multiple metabolic processes. These processes include photosynthesis and redox regulation, which lead to elevated levels of intracellular ROS and glutathione. Further, we show an impact of c-di-AMP on central carbon as well as on nitrogen metabolism. Effects on nitrogen metabolism are linked to reduced levels of the global nitrogen transcription regulator NtcA and highlighted by an imbalance of the glutamine to glutamate ratio as well as reduced metabolite levels of the arginine pathway. We further identified the HCO3- uptake systems, BicA and BCT1 as novel SbtB targets, in agreement with its broader role in regulating carbon homeostasis.