Discovery of ester-linked ubiquitylation of PARP10 mono-ADP-ribosylation in cells: a dual post-translational modification on Glu/Asp side chains
Discovery of ester-linked ubiquitylation of PARP10 mono-ADP-ribosylation in cells: a dual post-translational modification on Glu/Asp side chains
Bejan, D. S.; Lacoursiere, R. E.; Pruneda, J. N.; Cohen, M. S.
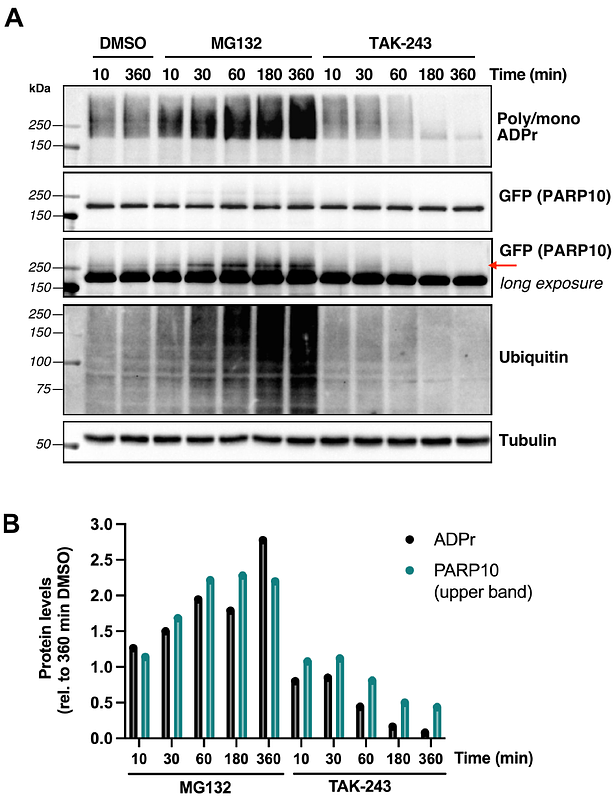
AbstractThe prevailing view on post-translational modifications (PTMs) is that amino acid side chains in proteins are modified with a single PTM at any given time. However, a growing body of work has demonstrated crosstalk between different PTMs, some occurring on the same residue. Such interplay is seen with ADP-ribosylation and ubiquitylation, where specialized E3 ligases ubiquitylate targets for proteasomal degradation in an ADP-ribosylation-dependent manner. More recently, the DELTEX family of E3 ligases was reported to catalyze ubiquitylation of the 3\'-hydroxy group of the adenine-proximal ribose of free NAD+ and ADP-ribose in vitro, generating a non-canonical ubiquitin ester-linked species. In this report, we show, for the first time, that this dual PTM occurs in cells on mono-ADP-ribosylated (MARylated) PARP10 on Glu/Asp sites to form a MAR ubiquitin ester (MARUbe). We term this process mono-ADP-ribosyl ubiquitylation or MARUbylation. Using chemical and enzymatic treatments, including a newly characterized bacterial deubiquitinase with esterase-specific activity, we discovered that PARP10 MARUbylation is extended with K11-linked polyubiquitin chains. Finally, mechanistic studies using proteasomal and ubiquitin-activating enzyme inhibitors demonstrated that PARP10 MARUbylation leads to its proteasomal degradation, providing a functional role for this new PTM in regulating protein turnover.


