3D-printed, Citrate-based Bioresorbable Vascular Scaffolds for Coronary Artery Angioplasty
3D-printed, Citrate-based Bioresorbable Vascular Scaffolds for Coronary Artery Angioplasty
Ding, Y.; Warlick, L.; Chen, M.; Taddese, E.; Collins, C.; Fu, R.; Duan, C.; Wang, X.; Ware, H.; Sun, C.; Ameer, G.
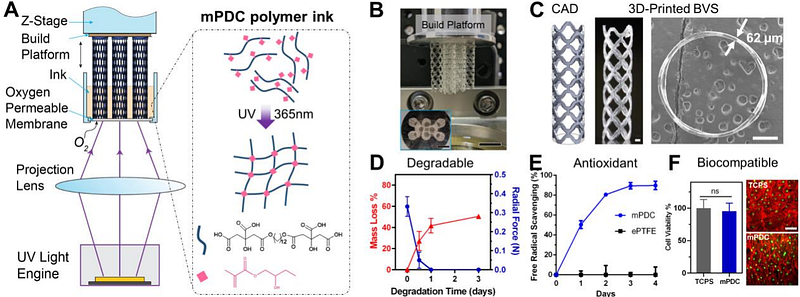
AbstractFully bioresorbable vascular scaffolds (BVSs) were designed to overcome the limitations of metallic drug-eluting stents (DESs). However, current polymer-based BVSs, such as Abbott Absorb, the only US FDA-approved BVS, struggle with increased strut thickness (150 m for Absorb) and exacerbated tissue inflammation, leading to inferior clinical performance compared to metallic DESs. Here we develop a drug-eluting BVS (DE-BVS) through the innovative use of photopolymerizable, citrate-based materials and high-precision additive manufacturing process. Bare BVS with a clinically relevant strut thickness of 62 m can be produced in a high-throughput manner, i.e. one BVS per minute. By modulating the coating polymer and structure, we achieve a controlled release of anti-restenosis drug of everolimus from DE-BVSs. We show the mechanical competence of DE-BVS and the successful deployment in swine coronary arteries using a custom-built balloon catheter delivery system. We further demonstrate that BVS and DE-BVS remain safe and effective to keep the vessel patency, induce limited inflammation, and facilitate the recovery of smooth muscle and endothelial tissues over 28 days implantation in swine coronary arteries. All these evaluated pre-clinical performances are largely comparable to the commercial XIENCETM DES (Abbott Vascular).