Modulating Hydrogel Stiffness Through Light-Based 3D Printing to Mimic Cardiac Fibrosis and Cardiomyocyte Dysfunction Using hiPSC-Derived Cells
Modulating Hydrogel Stiffness Through Light-Based 3D Printing to Mimic Cardiac Fibrosis and Cardiomyocyte Dysfunction Using hiPSC-Derived Cells
Sohn, S.; Momtahan, N.; Stevens, L. M.; Han, J.; Liu, Y.; Kiker, M. T.; Recker, E. A.; Page, Z. A.; Zoldan, J.
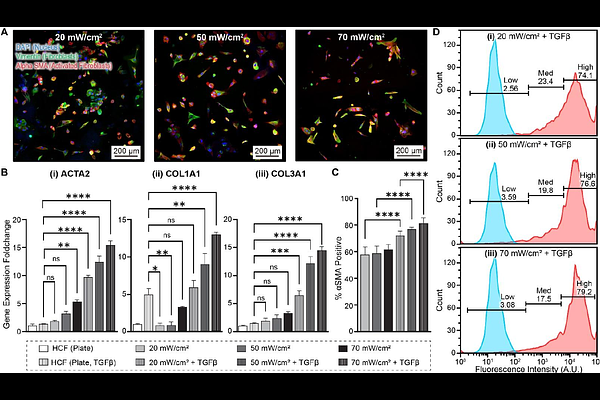
AbstractThe human heart\'s limited regenerative capacity is a significant barrier to addressing cardiovascular disease (CVD). This is particularly true for cardiac fibrosis, a form of CVD wherein the wound healing process has gone awry. In cardiac fibrosis, excessive scar tissue formation due to dysregulated remodeling of the heart\'s extracellular matrix (ECM) results in increased stiffness that reduces cardiac output and can lead to heart failure. This dysregulated ECM deposition is driven by activated cardiac fibroblasts, where cell substrate stiffness is known to play a role in cardiac fibroblast activation. New preclinical models that accurately recapitulate the behavior of activated cardiac fibroblasts are needed to better understand and treat cardiac fibrosis. To this end, we describe a model wherein human induced pluripotent stem cell (hiPSC)-derived cardiac fibroblasts (HCFs) are cultured on 3D printed hydrogels of tunable stiffness, fabricated using dosage controlled digital light processing (DLP). We demonstrate that our model can induce HCF activation in the absence of TGF{beta}, a key mediator of fibroblast activation, surpassing the activation levels seen with HCFs activated with TGF{beta} on protein-coated tissue culture plates. Furthermore, combining stiffer hydrogels with TGF{beta} recapitulates fibroblast activation similar to what is observed in native cardiac tissue. Lastly, by indirectly coculturing HCFs seeded and activated on these stiff hydrogels with hiPSC-derived cardiomyocytes, we demonstrate that the activated HCFs in our cardiac fibrosis model can impair cardiomyocyte function, mimicking the deleterious effects of cardiac fibrosis.