Peripheral T Helper Cells Dominate the Synovial CD4⁺ T Cell Compartment in Systemic Juvenile Idiopathic Arthritis and Are Shaped by IL-1β and IL-18
Peripheral T Helper Cells Dominate the Synovial CD4⁺ T Cell Compartment in Systemic Juvenile Idiopathic Arthritis and Are Shaped by IL-1β and IL-18
Dirks, J.; Fischer, J.; Harrer, L.; Bracaglia, C.; Prencipe, G.; Pardeo, M.; Magni-Manzoni, S.; Caiello, I.; Holl Wieden, A.; Girschick, H.; Kessel, C.; Morbach, H.
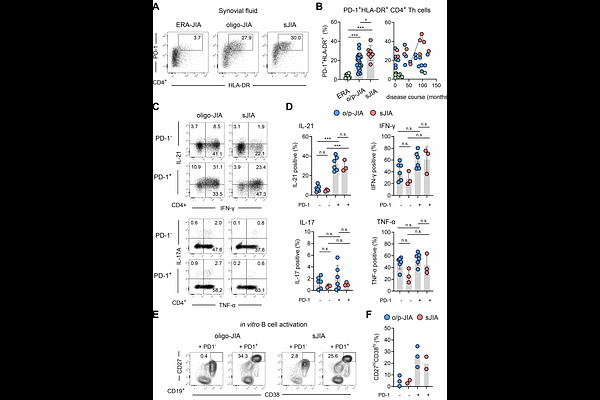
AbstractObjectives Systemic juvenile idiopathic arthritis (sJIA Still`s disease) is primarily driven by systemic autoinflammation but can progress to chronic arthritis, implicating dysregulated adaptive immunity. However, the molecular mechanisms within inflamed joints that link innate and adaptive immune activation remain poorly defined. This study aimed to define the transcriptional and clonal landscape of synovial CD4 T cells in sJIA and to identify inflammatory signals driving their differentiation. Methods Synovial fluid CD4 T cells from patients with sJIA and oligo/polyarticular JIA (o/p-JIA) were analyzed using flow cytometry, scRNA and TCR sequencing. Cytokine secretion and B helper function were assessed in vitro. The effects of IL-1{beta} and IL-18 on T cell differentiation were evaluated using bulk RNA sequencing and multiplex ELISA. Results sJIA joints harbored a dominant population of clonally expanded CD4 T cells with a T helper (Tph) cell state, marked by IL-21 and CXCL13 expression and robust B cell help. scRNA-seq revealed a heterogeneous CD4 T cell landscape, with transcriptional convergence of Tph and regulatory (Treg) programs particularly in sJIA. A subset of these cells exhibited molecular features consistent with differentiation from Tph precursors. In vitro, IL-1{beta} and IL-18 promoted Tph differentiation, aligning with the transcriptional profiles of expanded effector Tph cells observed in sJIA joints. Conclusion These findings identify Tph cell-driven adaptive immunity as a key feature of chronic arthritis in sJIA and link IL-1{beta} and IL-18 to Tph cell induction. This challenges the classical autoinflammatory paradigm and suggests that early cytokine-targeted therapy may modulate T cell fate and disease course.