Effects of a Cancer-Associated Mutation and Multiple SerinePhosphorylation on Poly(ADP-Ribose) Polymerase 2
Effects of a Cancer-Associated Mutation and Multiple SerinePhosphorylation on Poly(ADP-Ribose) Polymerase 2
Ghafari, M.; Cisneros, G. A.; Chatterjee, S.; Hughes, B.
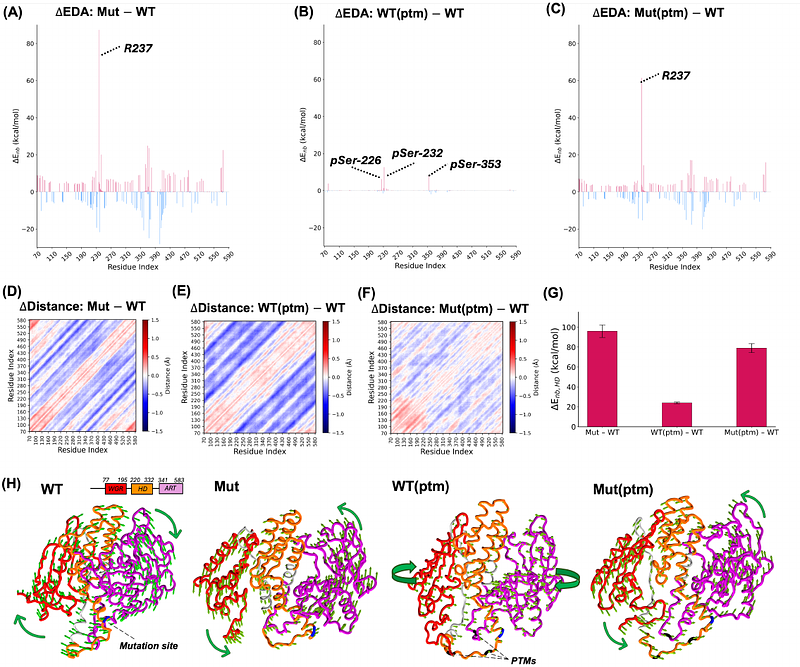
AbstractPoly [ADP-ribose] polymerase 2 (PARP2) plays a crucial role in DNA repair. A common single-nucleotide polymorphism (SNP) in the PARP2 gene, rs3093921, has been associated with pancreatic cancer and marginal zone lymphoma (MZL). This SNP results in a missense mutation, D235G, in the PARP2 protein. PARP2 is also reported to undergo post-translational modifications (PTMs), particularly phosphorylation at serine residues 226, 232, and 353. The C-terminal region of PARP2 includes the Trp Gly-Arg (WGR) and ADP-ribosyl transferase (ART) domains, and the helical subdomain (HD). The latter two, spanning residues 220 to 583, comprise the catalytic region of PARP2. The DNA-induced enzymatic activation of PARP2 is regulated by local destabilization of the HD domain. We used molecular dynamics (MD) simulations to investigate the impact of these three PTMs on both the wild-type (WT) and the mutant (D235G) forms of PARP2. Our simulations suggest that, while neither the cancer-associated mutation nor PTMs on their own significantly alter the ove all flexibility of residues within the HD domain, they cause notably greater deviations in the backbone structure of the HD domain compared to the WT. In addition, PTMs in the context of the mutation show reduced interactions between the mutation site and other protein regions, likely due to structural stabilization induced by the PTMs. Importantly, PTMs mitigate the structural disruption caused by the mutation, helping the mutant protein retain a WT-like conformation. Additionally, the HD domain contributes to maintaining PARP2 in its inactive state through its connection with the ART domain. However, the D235G mutation weakens this connection. While PTMs alone do not have a significant impact on this connection, the simultaneous presence of both the mutation and PTMs partially alleviates the disruptive effect of the mutation and leads to partial restoration of the connection between the HD and ART domains.