CryoEM Structure of the human THIK-1 K2P K+ Channel Reveals a Lower 'Y-gate' Regulated by Lipids and Anaesthetics
CryoEM Structure of the human THIK-1 K2P K+ Channel Reveals a Lower 'Y-gate' Regulated by Lipids and Anaesthetics
Rodstrom, K. E.; Eymsh, B.; Proks, P.; Hayre, M. S.; Madry, C.; Rowland, A.; Newstead, S.; Baukrowitz, T.; Schewe, M.; Tucker, S. J.
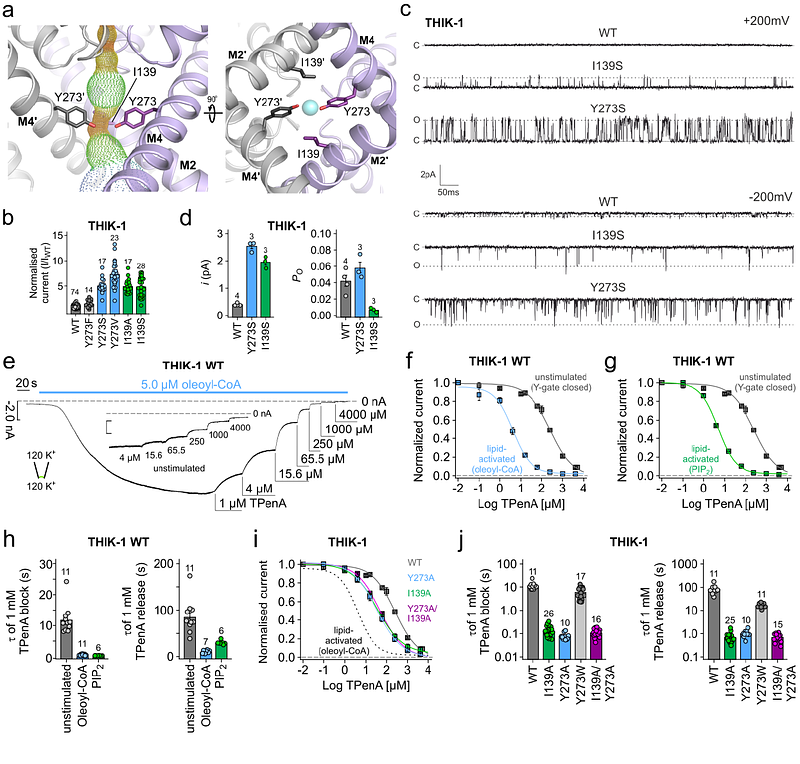
AbstractTHIK-1 (KCNK13) is a halothane-inhibited and anionic lipid-activated Two-Pore Domain (K2P) K+ channel implicated in microglial activation and neuroinflammation, and a current target for the treatment of neurodegenerative disorders such as Alzheimer\'s and Amyothropic Lateral Sclerosis (ALS). However, compared to other K2P channels, little is known about the structural and functional properties of THIK-1. Here we present a 3.16 Angstrom resolution cryoEM structure of human THIK-1 that reveals several unique features, in particular, a tyrosine in M4 (Y273) which contributes to a lower \'Y-gate\' that opens upon activation by several physiologically-relevant signalling pathways. We further demonstrate that binding of linoleic acid within a modulatory pocket adjacent to the filter also activates THIK-1, and that halothane inhibition involves a binding site within the inner cavity resulting in changes to the Y-gate. Finally, the extracellular cap domain contains positively-charged residues that line the ion exit pathway and which contribute to the unique biophysical properties of this channel. Overall, our results provide important insights into the structural basis of THIK1 function and identify distinct regulatory sites that expand its potential as a drug target for the modulation of microglial function.


