Cryo-electron tomography reveals the microtubule-bound form of inactive LRRK2
Cryo-electron tomography reveals the microtubule-bound form of inactive LRRK2
Chen, S.; Basiashvili, T.; Hutchings, J.; Murillo, M. S.; Suarez, A. V.; Louro, J. A.; Leschziner, A. E.; Villa, E.
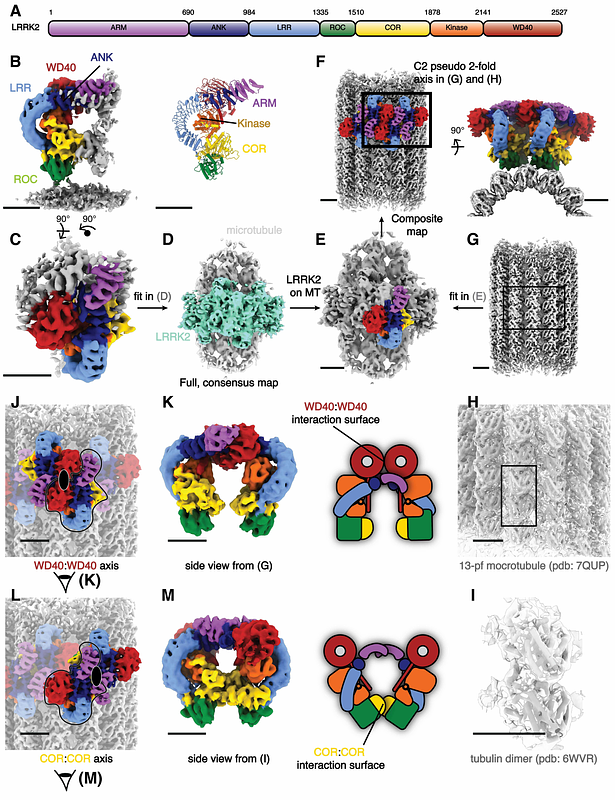
AbstractParkinson\'s Disease (PD) is the second most common neurodegenerative disorder. Mutations in leucine-rich repeat kinase 2 (LRRK2), a multi-domain protein containing both a kinase and a GTPase, are a leading cause of the familial form of PD. Pathogenic LRRK2 mutations increase LRRK2 kinase activity. While the bulk of LRRK2 is found in the cytosol, the protein associates with membranes, where its Rab GTPase substrates are found, and, under certain conditions, with microtubules. Integrative structural studies using single-particle cryo-electron microscopy (cryo-EM) and in situ cryo-electron tomography (cryo-ET) have revealed the architecture of microtubule-associated LRRK2 filaments, and that formation of these filaments required LRRK2\'s kinase to be in the active-like conformation. Whether LRRK2 can interact with and form filaments on microtubules in its autoinhibited state, where the kinase domain conformation is inactive and the N-terminal LRR domain covers the kinase catalytic site, was not known. Using cryo-ET, we showed that full-length LRRK2 can oligomerize on microtubules in its autoinhibited state. Both WT-LRRK2 and PD-linked LRRK2 mutants formed filaments on microtubules. While these filaments are stabilized by the same interfaces seen in the active-LRRK2 filaments, we observed a new interface involving the N-terminal repeats that were disordered in the active-LRRK2 filaments. The helical parameters of the autoinhibited-LRRK2 filaments are different from those reported for the active-LRRK2 filaments. Finally, the autoinhibited-LRRK2 filaments are shorter, suggesting they are less stable.