BtuJ1, a novel surface-exposed B12-binding protein in Bacteroidetes, functions as an extracellular vitamin reservoir that enhances fitness.
BtuJ1, a novel surface-exposed B12-binding protein in Bacteroidetes, functions as an extracellular vitamin reservoir that enhances fitness.
Abellon-Ruiz, J.; Pachecho-Gomez, R.; Watts, J.; Hart, A. J.; Hirt, R. P.; Basle, A.; van den Berg, B.
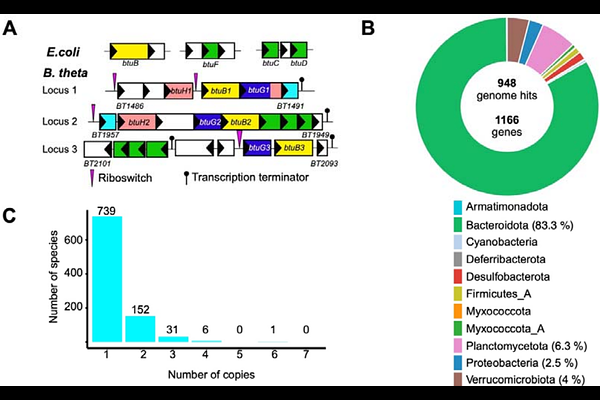
AbstractThe acquisition of vitamin B12 and related cobamides is a key determinant for the fitness of Bacteroidetes in the gut. Depending on the species, this uptake process relies on one to four transport systems centered on conserved core outer membrane (OM) complexes composed of the TonB-dependent transporter BtuB and the surface-exposed lipoprotein BtuG. Additionally, the surface-exposed lipoprotein BtuH, although not tightly associated with the BtuBG complex, contributes to cobamide uptake and provides a fitness advantage. Here, we report the functional and structural characterization of BtuJ1 from Bacteroides thetaiotaomicron (B. theta) , a novel B12-binding lipoprotein. Under limiting B12 conditions, BtuJ1 is the most abundant component among the three B12-transport systems encoded by B. theta. BtuJ1 is surface exposed and binds vitamin B12 and cobinamide (an intermediate in B12 biosynthesis) with low nM affinity, conferring a fitness advantage in B12-limited environments. In vitro B12 transfer experiments suggest a role for BtuJ1 as an extracellular reservoir for B12, expanding the functionalities of the diverse group of accessory OM proteins employed by Bacteroides to scavenge this essential cofactor in the competitive environment of the human gut.