MsyB-HU Interaction Modulates a Two-Tiered Bacterial Stress Response by Regulating DNA Supercoiling
MsyB-HU Interaction Modulates a Two-Tiered Bacterial Stress Response by Regulating DNA Supercoiling
Chen, H.; Yang, Y.; Chen, X.; Zhou, H.; He, Y.; Li, M.; Lin, W.; Wang, Y.; Matthews, S.; Yuan, S.; Wang, H.; LIU, B.
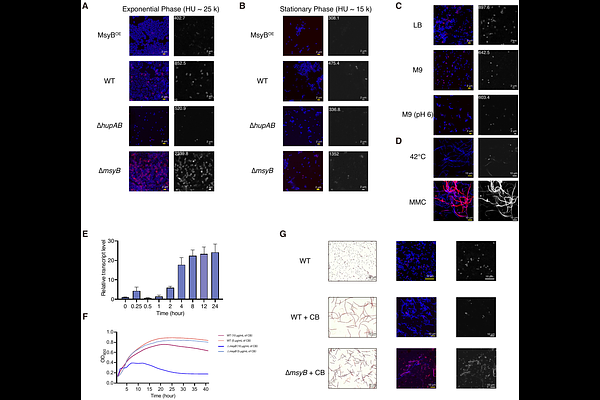
AbstractBacteria respond to stressful conditions through multiple pathways, including changes in gene expression patterns and mutations for adaptation. DNA supercoiling is a fundamental regulatory principle of bacterial gene expression, controlled antagonistically by DNA gyrase and topoisomerase I. Nucleoid-associated proteins, such as HU, also regulate supercoiling through interactions with DNA. Here, we identify Escherichia coli protein MsyB as a HU inhibitor and a {beta}-clamp binder. MsyB is fine-tuned under various stressful conditions, where it modulates the population of DNA-bound HU by forming a HU-MsyB complex. Consequently, the balance between DNA-bound HU and MsyB-bound HU adjusts the DNA supercoiling state, thereby influencing transcription for adaptation. Additionally, under prolonged starvation during the stationary phase, sustained high level of MsyB increases DNA exposure to damaging factors, acting as a damage inducer. The HU/MsyB/{beta}-clamp interaction suggests a model in which MsyB coordinates with HU and the {beta}-clamp to facilitate damage acquisition and error-prone DNA repair. Thus, MsyB-dependent supercoiling regulation represents a novel two-tiered bacterial stress response mechanism in gene expression and adaptive mutation. Our findings reveal a previously unrecognized regulatory mechanism of bacterial supercoiling, positioning HU as a promising antibiotic target.